Bio-inspired biomimetics can outperform natural coenzymes
'Better than Nature' compounds presented in the Journal of the American Chemical Society.
29 January 2016
The search for affordable, green biocatalytic processes is a challenge for chemicals manufacture. Not only is the use of natural coenzymes economically hardly viable, the moderate stability of these molecules thwarts their implementation in large scale catalytic processes that employ coenzyme recycling.
Now, in a joint JACS publication with researchers from TU Delft and the University of Manchester, Dr Tanja Knaus from the HIMS Biocatalysis group led by Dr Francesco Mutti describes the great potential of a range of synthetic biomimetics for replacing the natural coenzymes NAD(P)H in redox biocatalysis.

The new biomimetic coenzymes are inexpensive to manufacture and more stable than their biological counterparts, that are required as hydride source (reduced form NAD(P)H) or acceptor (oxidised form NAD+) in selective enzymatic reductive and oxidative reactions.
Outperforming natural counterparts
The HIMS Biocat researchers have investigated the performance of their biomimetic compounds with a wide range of oxidoreductase biocatalysts, in particular enzymes belonging to the family of the "ene"-reductases. These enzymes catalyse the asymmetric reduction of activated alkenes and their activity can be exploited for the synthesis of high value chemical products such as pharmaceuticals and agrochemicals.
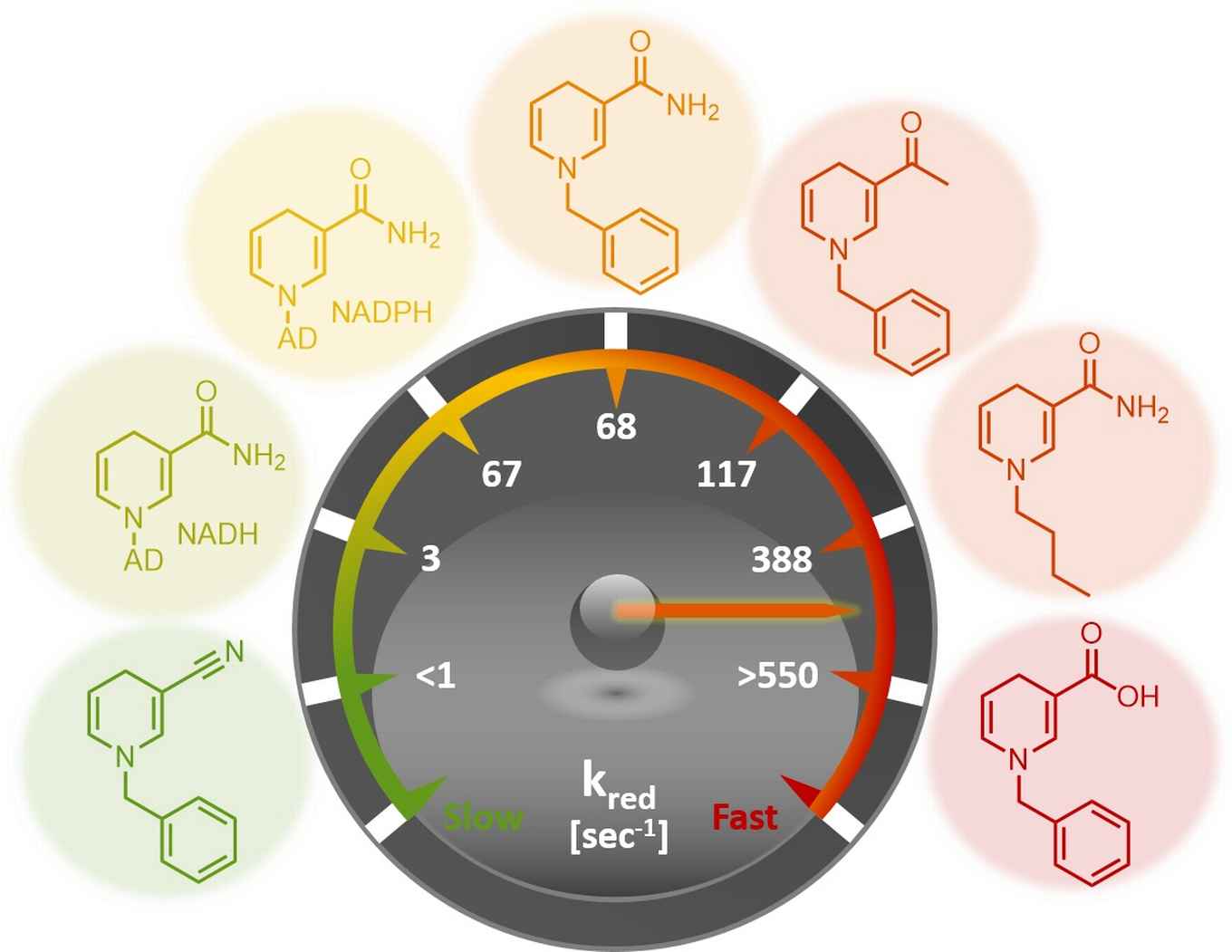
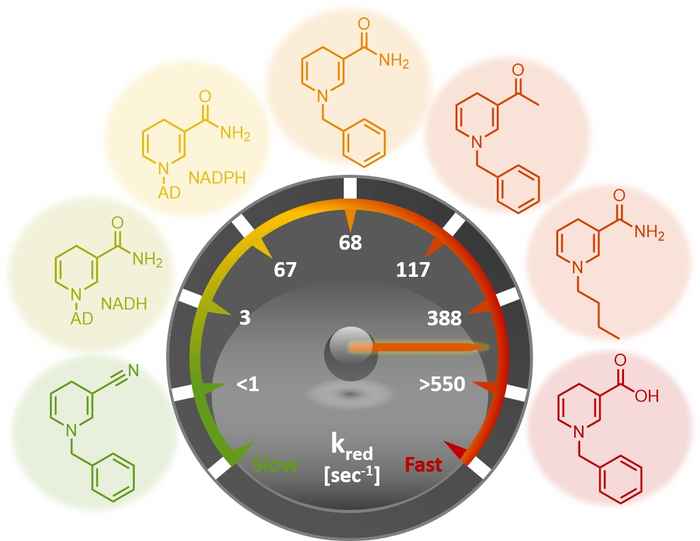
The researchers elucidated the performance of the biomimetics through steady-state and rapid-reaction kinetics as well as the analysis of the X-ray crystal structures of the biomimetics in complex with the ene-reductase XenA. The analysis of the kinetic parameters shows that, in selected cases, these biomimetics outperform the natural coenzymes. That laboratory-based designs can outperform those available in Nature is quite notable.
Moreover, the biomimetics have been successfully applied to the asymmetric catalytic reduction of activated alkenes. It is noteworthy that the biomimetics could be employed in catalytic amount as they were recycled in situ at the expense of formate.
A future for redox biocatalysts
The HIMS Biocat researchers conclude that the implementation of these synthetic biomimetics - as well as the design of more sophisticated analogues capable of operating with a variety of other oxidoreductases - will facilitate the use of redox biocatalysts in chemicals production and thereby transform the use of oxidoreductases more widely in industrial biocatalysis.
They expect that the "Better-than-Nature" biomimetics can find widespread application in fine and specialty chemicals production by harnessing the power of high stereo-, regio-, and chemoselective redox biocatalysts and enabling reactions under mild conditions and at low cost.
The research of the HIMS-Biocat group was carried out in collaboration with the biocatalysis group of TU Delft (Dr C. P. Paul and Dr. F. Hollmann) and the enzymology group of the University of Manchester (Prof. N. S. Scrutton).
Article:
Tanja Knaus, Caroline E. Paul, Colin W. Levy, Simon de Vries, Francesco G. Mutti, Frank Hollmann, and Nigel S. Scrutton: Better than Nature: Nicotinamide Biomimetics That Outperform Natural Coenzymes Journal of the American Chemical Society 2016 138 (3), 1033-1039 DOI:10.1021/jacs.5b12252
Other recent highlights for the HIMS-Biocat group
Angewandte Chemie
The implementation of a one-pot multi-enzyme cascade in a single bacterial whole cell system has been recently published in Angewandte Chemie Int. Ed..

In this research, a P450 monooxygenase, an alcohol dehydrogense and an omega-transaminase have been cloned in the same host organism to carry out the formal regio- and stereoselective amination of benzylic C-H bonds. This research was performed in collaboration with the University of Manchester (Prof. S. L. Flitsch) and was featured on the inside cover of Angewandte.
P. Both, H. Busch, P.P. Kelly, F.G. Mutti, N.J. Turner, S.L. Flitsch: Whole-Cell Biocatalysts for Stereoselective C−H Amination Reactions, Angew. Chem. Int. Ed. 2016, 55, 1511. DOI:10.1002/anie.201510028
Nature Chemistry
Dr. Jian-bo Wang and Prof. Manfred T. Reetz from the “Max-Planck-Institut für Kohlenforschung” in Mülheim (Germany) have highlighted the research conducted by the HIMS-Biocat group on “biocatalytic hydrogen-borrowing amination of alcohols” in the section News and Views of the December issue of Nature Chemistry.
Jian-bo Wang & Manfred T. Reetz: Biocatalysis: Chiral cascades, Nature Chemistry 7, 948–949 (2015) DOI:10.1038/nchem.2408.