Formation of amyloid fibrils highly susceptible to salt concentration
New findings can help explain contradictory research findings regarding Parkinson's and other diseases.
6 February 2017
The research was carried out by PhD students Steven Roeters (Van 't Hoff Institute for Molecular Sciences, UvA, under the supervision of Prof. Sander Woutersen ) and Aditya Iyer (AMOLF, under the supervision of Prof. Vinod Subramaniam, VU). They studied the aggregation of the intrinsically disordered protein alpha-synuclein (αS) into amyloid fibrils, a process known to be involved in neuronal cell death in Parkinson’s disease. Using a combination of techniques, among which atomic force microscopy, UV-circular dichroism CD, X-ray diffraction and sophisticated 2D infrared spectroscopy, they were able to show that the structure of these αS fibrils is highly sensitive to the salt concentration (ionic strength) during the aggregation process.
Fifty disorders
Currently approximately fifty disorders are known to be related to amyloid fibril formation, including Alzheimer’s and Parkinson’s disease, and type-II diabetes. Since recent studies show that subtle difference in fibril structure can have varied effects on cell death, the researchers argue that the observed high sensitivity to ionic strength can have significant physiological implications. They also anticipate that the observed high sensitivity of αS to aggregation conditions can contribute to the relatively frequent occurrence of contradicting findings in the research field. They expect that their proposed mechanism will be relevant to other amyloid forming proteins.
Dramatic changes in morphology
The researchers observed significant differences between the structures of fibrils aggregated in low-salt and in high-salt buffers.
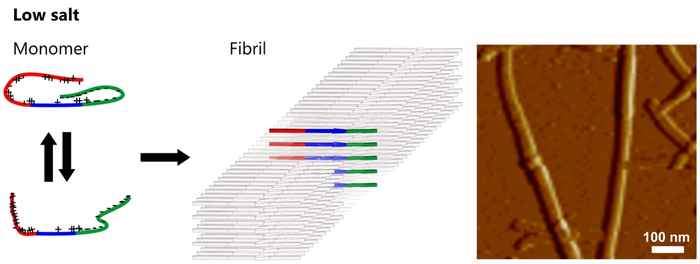
In low-salt buffers ([NaCl] ≤ 25 mM) the αS molecules form hydrogen-bonded intermolecular β-sheets that are loosely packed in a parallel fashion. The resulting fibrils have a ribbon-like morphology.
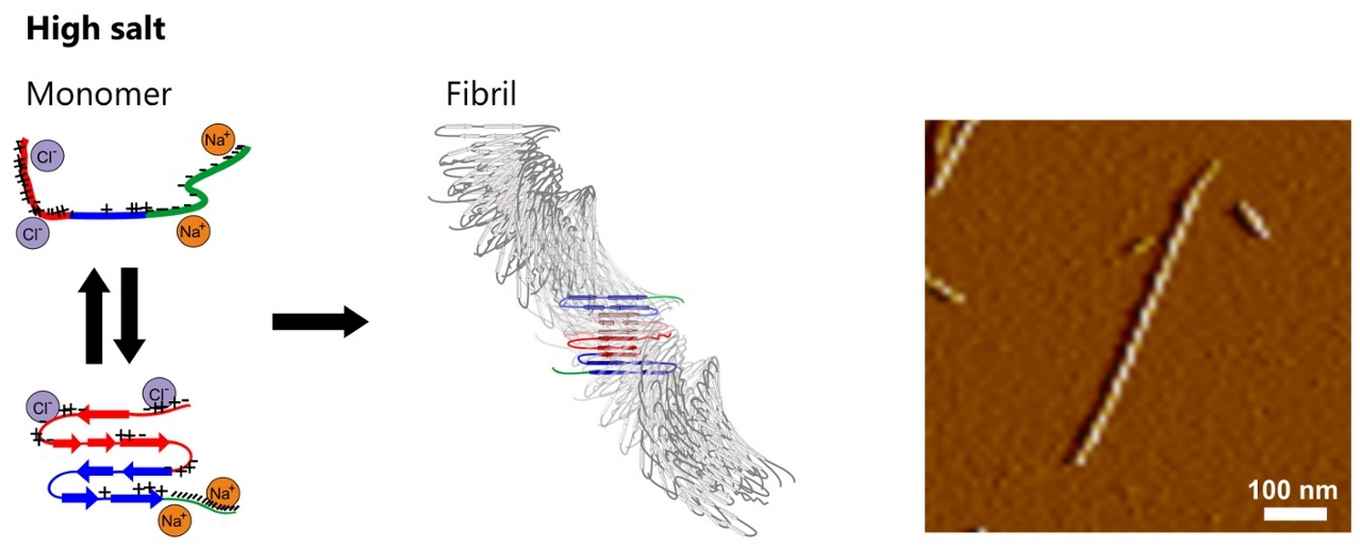
This spatial structure differs fundamentally from fibrils that aggregate in high-salt buffers (including those prepared in buffers with a physiological salt concentration):
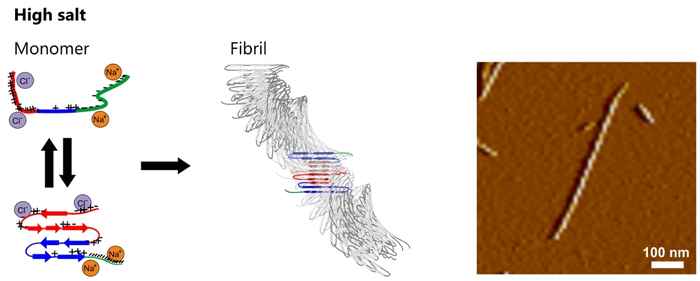
Here the αS molecules are more tightly-packed in an antiparallel intramolecular fashion and form a structure characterized by two twisting stacks of approximately five hydrogen-bonded intermolecular β-sheets each. This results in fibrils with a twisted rod-like morphology.
Coulombic interaction
The authors explain the salt sensitivity by its influence on the monomer structure of the αS protein. In the absence of salt the interaction between the oppositely charged tails can stabilise a folded conformation of the protein. A salt environment strongly reduces this Coulombic interaction, leading to an unfolded conformation which exposes the previously shielded hydrophobic central region of the protein. In order to minimize the hydrophobic exposure, the protein folds into an antiparallel β-sheet conformation with a relatively high aggregation propensity.
Publication
Steven J. Roeters, Aditya Iyer, Galja Pletikapić, Vladimir Kogan, Vinod Subramaniam & Sander Woutersen: Evidence for Intramolecular Antiparallel Beta-Sheet Structure in Alpha-Synuclein Fibrils from a Combination of Two-Dimensional Infrared Spectroscopy and Atomic Force Microscopy. Scientific Reports 7, Article number: 41051 (2017) doi:10.1038/srep41051