The unexpected supramolecular chemistry of nitrate anions
Nitrate anions can, surprisingly, act as Lewis acids
21 February 2017
Chemistry is all about electrons. The behaviour of atoms and molecules (their acidity, reactivity, molecular configuration and so forth) depends largely on the distribution of their electrons. In general chemists are able to make fairly accurate predictions using a comprehensive set of rules containing the 'chemical rationale' derived from the electronic properties. However, once in a while reality defies this 'chemical common sense'.
A striking example has just been published in Nature Communications by HIMS researcher Dr Tiddo J. Mooibroek in collaboration with scientists from the Balearic Islands (Spain). They argue that under certain circumstances nitrate anions (NO3–) can display a counterintuitive Lewis acidity: the nitrate anions can act as electron acceptors while they are generally considered as electron donors.
New interpretation of data
The researchers show that the nitrate anions can interact favorably with electron rich entities in the solid state. However, their reasoning seems valid for NO3– in solution as well. As nitrate anions are very common in chemistry and biology, the researchers anticipate that their findings may serve as a (retrospective) guide to interpret data where nitrate anions are involved.
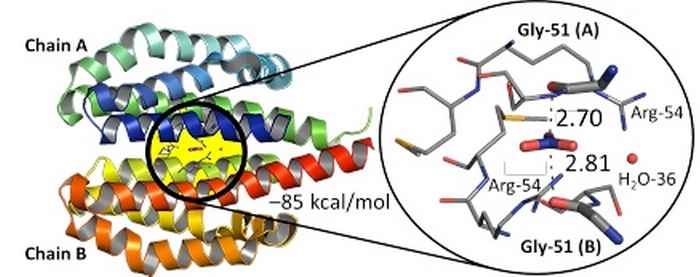
3EZH protein structure from the Brookhaven Protein Databank with a zoom-in of the nitrate ligand's binding pocket. Image: Tiddo J. Mooibroek, HIMS.
Examples are ortho nitrate (NO42-) formation; cases where NO3– anions may be a structural determinant (such as in 3EZH proteins); or transport and recognition phenomena involving this ubiquitous anion.
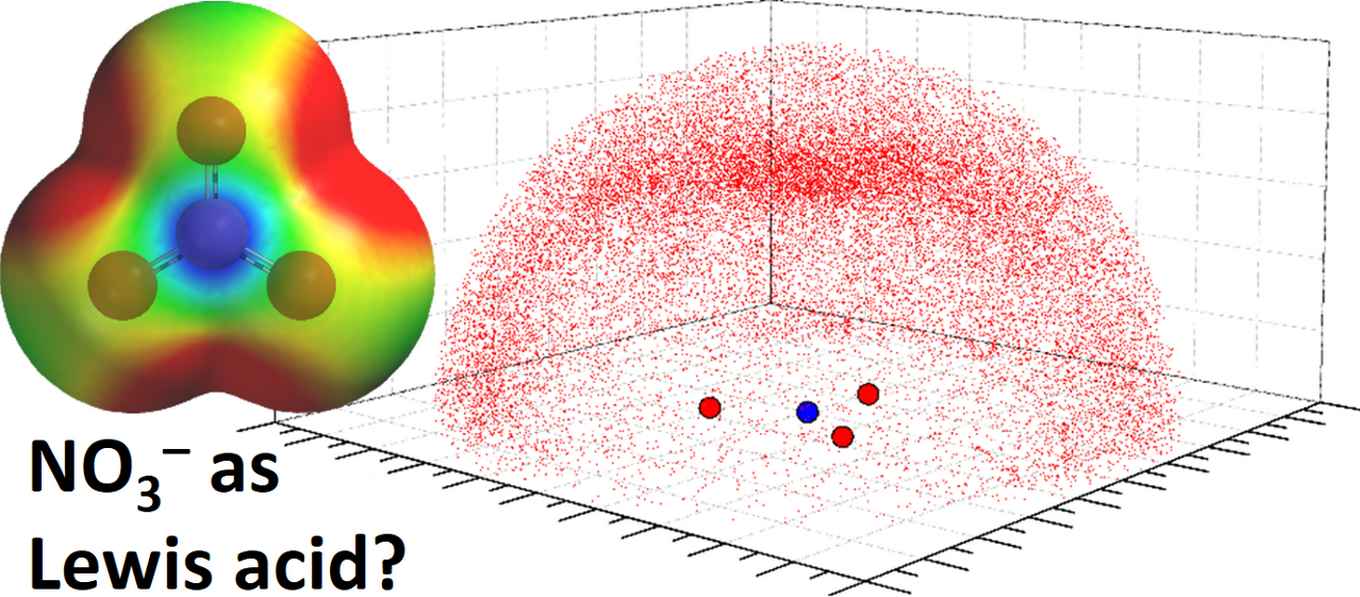
A Lewis acidic site emerging on the nitrogen atom
By means of computational calculations the researchers show that when the nitrate’s charge is sufficiently dampened by resonating over a larger area, a Lewis acidic site emerges on the nitrogen atom. Computations further predict that in such circumstances electron rich partners (e.g. anions or lone-pair electrons) interact favorably with the Lewis acidic site.
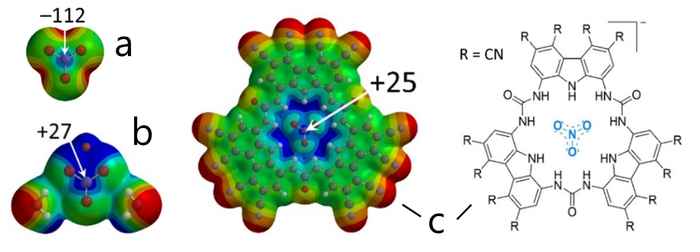
Calculated Molecular Electrostatic Potential maps (MEPs) with color codes representing more negative (red) to more positive (blue) potentials. Combining a nitrate anion (a) with electron-drawing moieties such as a lithium ion and two water molecules [LiNO3∙2H2O] (b) or cyanide-substituted trisurea macrocycles (c) results in an electron-deficient, Lewis-acidic site (the interaction energy of [c∙Cl]2– was calculated to be –31.6 kcal/mol-1). Images: HIMS, Tiddo J. Mooibroek.
Experimental support for this idea was found by surveys of the solid state structures contained within the Cambridge Structural Database (CSD) and the Brookhaven Protein Data Bank (PDB). These studies revealed geometric preferences of some oxygen and sulfur containing entities around a nitrate anion that are consistent with an interaction between the Lewis acidic site on N and the electron rich O/S. Computations of selected examples reveal donor-acceptor orbital interactions that confirm the counterintuitive Lewis acidity of nitrate.
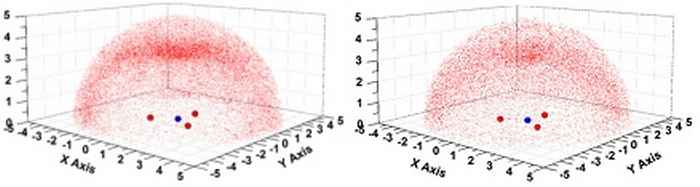
Distribution of sp2 O-atoms around uncoordinated NO3– anions as observed within the CSD (left) and PDB (right). Images: Tiddo J. Mooibroek, HIMS.
Unique set of properties
Contemplating what other anions might be capable of displaying such behavior (using other atoms than hydrogen) the researchers argue that nitrate actually has a rather unique set of properties that sets it apart from other common anions in this respect: NO3– is fairly polarized and further polarizable, not so charge-dense, and nitrate is flat, rendering the Lewis acidic site sterically accessible.
Article
Antonio Bauzá, Antonio Frontera and Tiddo J. Mooibroek: NO3– anions can act as Lewis acid in the solid state. Nature Communications, published online 21 February 2017, DOI: 10.1038/NCOMMS14522