Record breaking reactivity of new iron catalyst for synthesis of cyclic amines
Publication in Journal of the American Chemical Society
20 April 2017
Another important feat of the new catalyst is that it can be efficiently recycled. The research, funded by the European Research Council and supervised by Dr Jarl Ivar van der Vlugt, was performed by postdoctoral research fellow Dr. Bidraha Bagh and several of his colleagues of the Homogeneous, Bio-inspired and Homogeneous research group, part of the UvA Research Priority Area Sustainable Chemistry. Their breakthrough has recently been published by the Journal of the American Chemical Society.
Selective Catalytic Intramolecular C(sp3)−H Amination
The development of atom-efficient methodologies to install C−N bonds via intra- or intermolecular C(sp3)-H amination protocols has significantly advanced in the last decade. In particular the intramolecular C(sp3)−H amination has found extensive applications to construct important N-heterocycles. Nitrene insertion into a C(sp3)-H bond is a very attractive protocol, but typical procedures to generate these nitrenes often involve the use of directing groups, pre-oxidation of substrates or external chemical oxidants, leading to poor atom economy and waste generation.
Previous research by the same group has demonstrated that the use of a reactive organic platform (a ‘redox-active ligand’) is crucial to persuade the noble metal palladium to facilitate single-electron transfer reactivity and to enable modest catalytic activity for the conversion of readily available aliphatic azides into valuable N-heterocycles (see J. Am. Chem. Soc. 2014, 136, 11574 and press-release on the HIMS website). Although the ligand-induced single-electron transfer was found to trigger the activation of these easily accessible organic substrates, the initial Pd-system proved too sensitive for significant improvement of the catalytic performance.
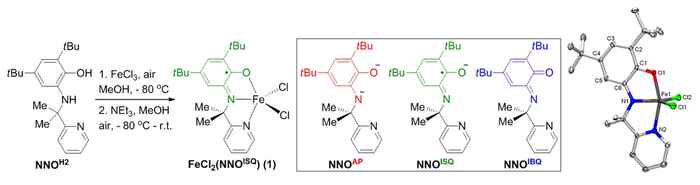
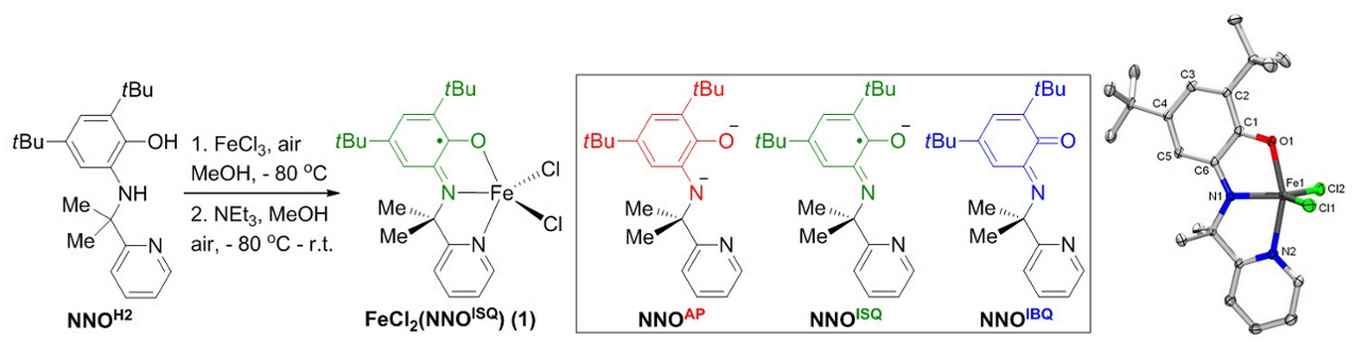
Synthesis of Fe-catalyst 1 is straightforward from FeCl3 and the NNOH2 ligand previously developed by the group. This NNO-platform can be present in three different oxidation states. X-ray crystallography confirms the presence of the ligand radical NNOISQ state in complex 1. Image: HIMS. (Click to enlarge).
In the current work, the team demonstrates that an FeIII-system supported by the same exact ligand platform outperforms all reported homogeneous and immobilized transition metal systems for the direct C(sp3)-H amination of aliphatic azides to N-heterocycles, with turnover numbers that are up to two orders of magnitude higher than previously established.
Easily assembled
The very efficient, bench-stable and easy-to-handle recyclable Fe-catalyst is easily assembled from an FeIII-chloride precursor in combination with the proprietary redox-active NNOH2 ligand, resulting in an air-stable crystalline solid that has been fully characterized. The team has tested various types of aliphatic azides for the efficient conversion to the corresponding N-heterocycle products, providing good to excellent selectivity.
The Fe-system allows for easy recollection after the reaction is complete, allowing for full recovery and re-use. Although initial kinetic information is available for this unique system, the precise mode of action for this Fe-system is still under investigation, as the presence of a redox-active metal, ligand and substrate complicate matters significantly.
Article
B. Bagh, D.L.J. Broere, V. Sinha, P.T. Kuipers, N.P. van Leest, B. de Bruin, S. Demeshko, M. A. Siegler, J.I. van der Vlugt, Catalytic Synthesis of N-Heterocycles via Direct C(sp3)–H Amination Using an Air-Stable Iron(III) Species with a Redox-Active Ligand J. Am. Chem. Soc., 2017, 139 (14), pp 5117–5124
DOI: 10.1021/jacs.7b00270