Amsterdam chemists synthesize molecular pretzels
Towards synthetic lasso peptides
25 May 2017
![quasi[1]catenane](/binaries/_ht_1701916622500/1360x0-jpg/content/gallery/onderzoek/hims/synthetic-organic-chemistry/catenaaneng.jpg)
The Nature Communication article is the crowning achievement of a five-year research effort at the Synthetic Organic Chemistry research group of professor Jan van Maarseveen, with PhD student Luuk Stemers in the lead. He has developed a method that paves the way for synthesis of so-called lasso peptides.
Lasso peptides are small proteins that, as their name indicates, consist of a molecular ‘loop’ around a molecular ‘rope’. They were first isolated from bacteria at the turn of the current century. Recently, DNA analysis has revealed that lasso peptides are quite common in the realm of bacteria. Their biological function is to act as an antibiotic against other micro-organisms, which makes them a potential new class of antibiotics.
Complex molecules
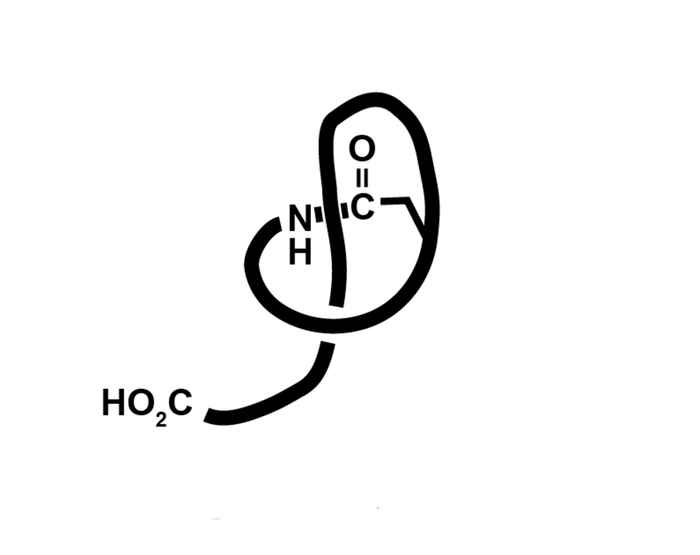
The fact that 15 years after the discovery of lasso-peptides synthetic chemists have not yet been able to develop a strategy leading to their unique molecular architecture underpins the complexity of these molecules.
The bottleneck here is that the rope is usually tightly bound within the loop. This distinguishes lasso peptides from rotaxanes for which Scottish chemist Sir Fraser Stoddart shared the Nobel prize for chemistry last year. During rotaxane synthesis the rope is ‘pulled’ through the loop.
Since this is impossible for lasso peptide synthesis, the Amsterdam chemists used a different approach, forcing the loop to close in the right place around the rope. This turned out to be quite an undertaking. Eventually Luuk Stemers managed to create a molecular scaffold assisting the synthesis in such a way that the loop correctly forms around the rope.
Powerful method
The new synthesis method is a major step forward in the synthetic route towards functional lasso peptides. To demonstrate the power of the method Stemers applied his scaffold to also force both ends of the rope to form a second loop.
![quasi[1]catenane](/binaries/_ht_1701916622631/700x0-jpg/content/gallery/onderzoek/hims/synthetic-organic-chemistry/catenaaneng.jpg)
This resulted in the synthesis of a whole new class of pretzel-like molecules that the Amsterdam researchers coined quasi[1]catenanes. ('Real' catenanes consist of two loosely intertwined molecular ring-like structures. The French chemist Jean-Pierre Sauvage developed catenanes and shared the Nobel prize with Stoddard, and Dutch chemist Ben Feringa.)
The next step in the research effort of the Amsterdam researchers towards lasso peptide synthesis will be to introduce easily breakable bonds in the quasi[1]catenane, so that the rings can be unlocked.
Article
Luuk Steemers, Martin J. Wanner, Martin Lutz, Henk Hiemstra & Jan H. van Maarseveen: Synthesis of spiro quasi[1]catenanes and quasi[1]rotaxanes via a templated backfolding strategy. Nature Communications, published online 25 May 2017. DOI: 10.1038/ncomms15392.