Amsterdam chemists make bicycle-like molecular drive
Novel switches for molecular machines operating under confined conditions
7 February 2018

The research will be appear in the coming print issue of Angewandte Chemie International Edition (to be published 12 February). The accompanying front cover illustration highlights the importance of the work.
Switching using only a bare minimum of volume
Molecules that can be switched by light to change their structure are key building blocks for photoresponsive molecular nanotechnology. A major drawback of many currently available molecular switches is that they require a relatively large free volume to reverse between their two structural states. Prototypical examples are molecules in which isomerization of a double bond occurs, such as the rotor molecules of Nobel Laureate Ben Feringa.In many practical applications, for instance in catalysis, drug delivery or molecular computers, there simply is not enough space for such large-scale motion. Finding new chemical motifs that enable switching using only the bare minimum of volume is therefore of great relevance to this rapidly emerging field.
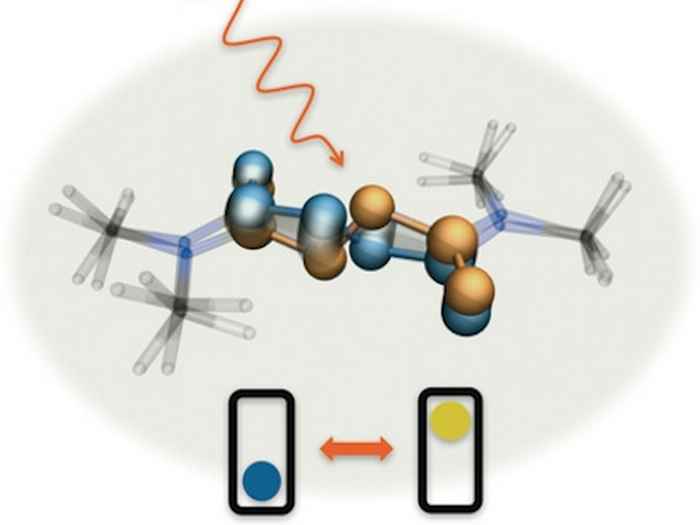
New switches
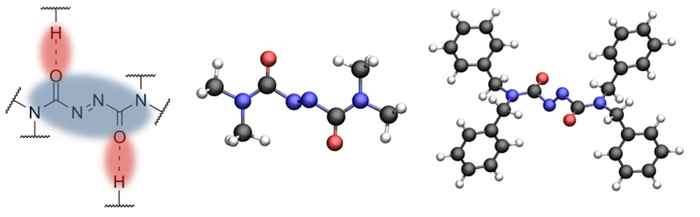
Recently, Prof. Jose Berna of the University of Murcia has proposed a new class of azodicarboxamide-based molecular switches. These are derived from a modification of the azo-moiety in azobenzene - one of the most widely employed components in 'light switchable' materials. Since the new systems are - in contrast to azobenzenes - no longer planar, it was expected they would exhibit different types of motion upon irradiation with light. Until now, however, studies of the actual motion taking place remained out of reach.
Pedalo-type of motion induced by light
To investigate the exact mode of operation of the azodicarboxamide-based molecular switches, Dr Saeed Amirjalayer* at the University of Amsterdam set out to measure their vibrational frequencies using extremely short pulses of infrared light (with a duration of less than a trillionth of a second). These frequencies are a fingerprint of the molecular structure and thus offer a direct means to establish exactly how the molecule changes its structure after being activated by light.
As it turned out these switches indeed exhibit a switching mechanism that is completely different when compared to the standard switches. Where the latter exhibit large-scale rotation around one bond, the new molecules operate like the bottom bracket and pedals of a bicycle. They do not, however, perform a full rotation, but move back and forth. Using advanced quantum chemical calculations it was established that the molecules become planar by light absorption and crank back as they return to their ground state.
New opportunities for new switches
The striking characteristic of the pedalo motion is that it is accompanied by minimal displacements of the atoms involved. The molecule thus stays more or less fixed in space and needs only a minimal switching volume. This offers opportunities for applications where motion at the molecular level is heavily restricted, such as in the solid state, on surfaces, or upon embedding in polymers.
* Dr Saeed Amirjalayer currently is group leader at the University of Münster in Germany.
Reference
Saeed Amirjalayer, Alberto Martinez-Cuezva, Jose Berna, Sander Woutersen, and Wybren Jan Buma: Photo-induced pedalo-type motion in an azodicarboxamide-based molecular switch. Angewandte Chemie International Edition, 2018, 57, 1792; DOI: 10.1002/anie.201709666.
Cover image: DOI 10.1002/anie.201800060