Dutch-Chinese cooperation in supramolecular catalysts
9 February 2018
With the program Cooperation China (NSFC), NWO stimulates cooperation between Dutch and Chinese researchers. The recent call concerns four projects in the field of supramolecular chemistry and catalysis. Both the Netherlands and China perform high-quality and highly cited scientific research in this area.
Advanced hydrogenase mimics
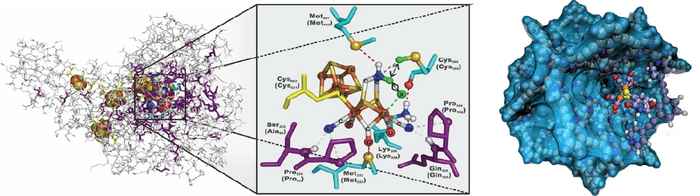
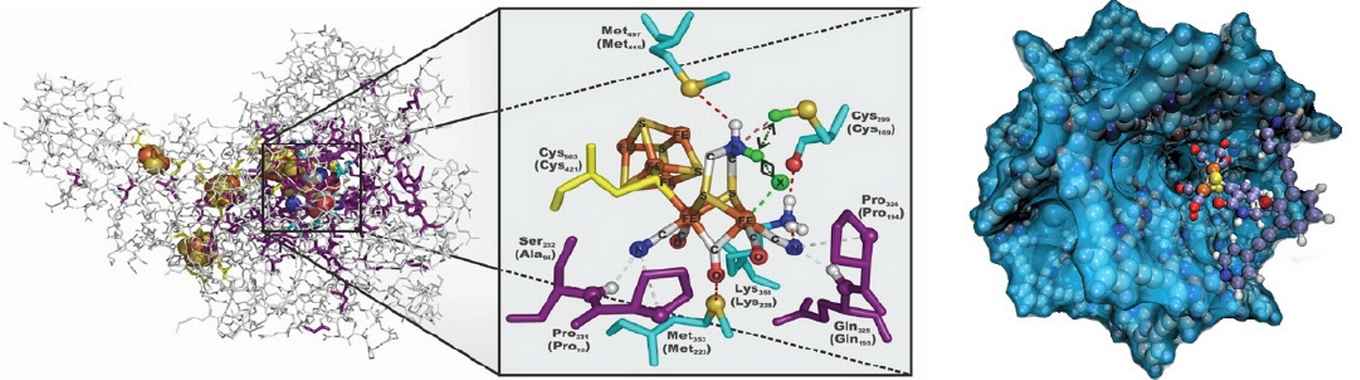
The project of Prof. Reek, which also includes dr.ir. Jarl-Ivar van der Vlugt, focuses on advanced synthetic catalysts inspired by the natural hydrogenase enzyme. Nature is able to very efficiently generate hydrogen using this enzyme. Although chemists have made many attempts to mimic these enzymes, comparably high efficiencies have remained out of reach.
Together with their Chinese partners, Reek and Van der Vlugt now want to realize improved, more advanced mimics, using self-assembling complex peptide structures.
On the Chinese side Prof. Li-Zhu Wu from the Technical Institute of Physics and Chemistry (Beijing) will cooperate with Prof. Cheng He of the State Key Laboratory of Fine Chemicals at Dalian University of Technology (Dalian).
Combination with light harvesting components
During this project strategies will be developed to place well-defined Fe2S2 hydrogenase mimics inside M12L24 nanospheres that are internally decorated with functionalized peptide chains. This will allow to study exactly how the peptide environment has an influence on crucial catalyst properties such as rate and overpotential at which the mimic starts to produce hydrogen.
The spheres will also be combined with light-harvesting components for light driven proton reduction. Detailed photophysical studies will unravel relate important parameters such as charge separation and recombination, to the efficiencies of photocatalysis. In a related strategy, the polypeptides and hydrogenase mimics will be brought together using MOF synthesis, which allows the generation of novel functional devices.