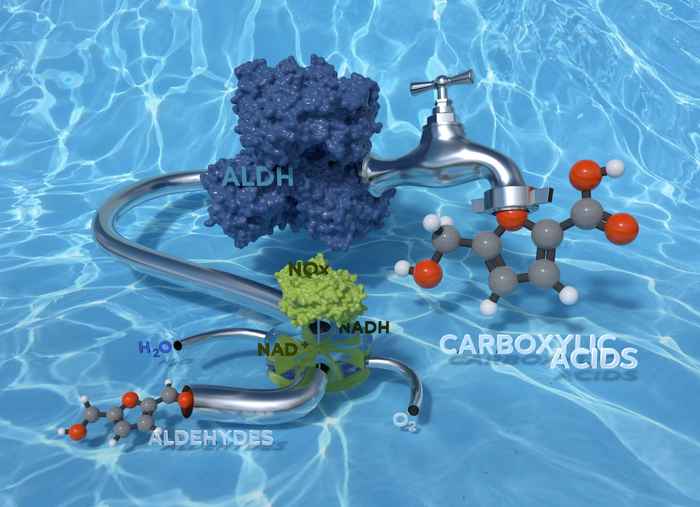
Sustainable and very selective biocatalytic conversion of aldehydes to carboxylic acids
Front cover of Green Chemistry features HIMS research
30 August 2018

The oxidation of aldehydes to carboxylic acids is an important and industrially relevant chemical reaction, for instance with respect to the synthesis of pharmaceuticals and bio-based polymers. Current oxidation procedures require the abundant use of toxic chemical reagents and often produce unwanted side-products.
In search for improvement of the environmental footprint for aldehyde oxidation, various novel synthetic methods have been investigated. Until now, however, no economically viable procedure has been developed that is based on environmentally benign reagents and/or solvents, and that combines an elevated productivity with a perfect selectivity (meaning that only desired aldehyde functional groups are oxidized, thus minimizing or even completely avoiding the formation of unwanted side products).
Benign biocatalysis
As an interesting 'green' alternative, biocatalytic, enzyme-based methods for the oxidation of functional groups display mild reaction conditions (ambient temperature and atmospheric pressure) in an aqueous environment, and they generally achieve very good selectivities. Furthermore, they can utilize molecular oxygen as a benign oxidant.
The HIMS research team led by Dr Francesco Mutti now has successfully explored the use of aldehyde dehydrogenase enzymes for the oxidation of aldehydes to carboxylic acids. In an article recently accepted by the high-impact RSC journal 'Green Chemistry', the researchers present a study on three recombinant aldehyde dehydrogenases originating from bovine lenses and the bacteria Escherichia coli and Pseudomonas putida. For regeneration of the catalytic NAD+ coenzyme, they applied the H2O forming NAD-oxidase from Streptococcus mutans. The final bio-oxidation runs in aqueous phosphate buffer, under mild reaction conditions (40 °C and atmospheric pressure) and consumes only dioxygen from air as the oxidant.
Extensive study
To investigate the potential of the three enzymes, the researchers performed an extensive study where they tested sixty-one structurally diverse aldehydes. The majority of these substrates (aliphatic, aryl aliphatic, benzylic-, hetero-aromatic and bicyclic aldehydes) were converted with yields of well over 60% and in many cases even over 99%. The only exceptions were some ortho-substituted benzaldehydes and two bicyclic heteroaromatic aldehydes.
In all cases, the chemoselectivity was perfect: no other product was detected except the expected carboxylic acid. This means that other oxidizable functionalities (such as the hydroxyl moiety, alkene groups, aryl groups, and sulphur as well as nitrogen heteroatoms) remained untouched.
Whole cells
Since for practical applications the use of whole cells rather than purified enzymes is to be preferred, avoiding time consuming and costly purification steps, the researchers also investigated the bio-oxidation with E. coli lyophilised whole cells as well as resting cells. It turned out that supplementation of NAD+ coenzyme and NOx recycling enzyme can be omitted in some cases as the microbial host produces sufficient amount of coenzyme, which can be recycled by endogenous E. coli enzymes. In particular, bio-based 5-(hydroxymethyl)furfural was converted into 5-(hydroxymethyl)furoic acid, in a two gram-scale reaction with perfect chemoselectivity and 61% isolated yield. 5-(hydroxymethyl)furoic acid finds application as building block for the production of bio-based polymers and pharmaceuticals.

The researchers conclude that aldehyde dehydrogenases have the potential to become the first choice for chemoselective oxidation of aldehydes into carboxylic groups. Their biocatalytic method is particularly attractive for the oxidation of aldehyde moieties within molecules possessing further oxidizable groups. Future research will focus on improving the enzymes tolerance to substrate concentration and long-term stability in order to enable even broader application of these enzymes.
Publication details
Knaus, Tanja & Tseliou, Vasilis & D Humphreys, Luke & Scrutton, Nigel & Mutti, Francesco. (2018). A biocatalytic method for the chemoselective aerobic oxidation of aldehydes to carboxylic acids. Green Chemistry. DOI: 10.1039/C8GC01381K
Front cover: DOI: 10.1039/C8GC90082E