Laboratory Astro Chemistry

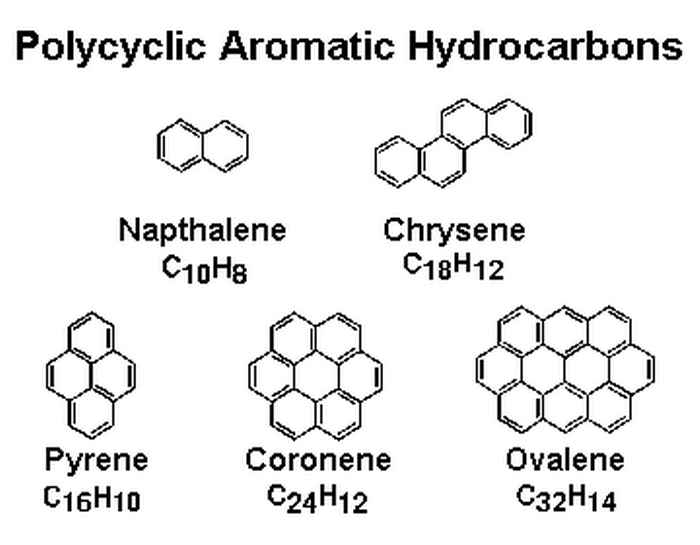
During a supernova, when the massive star explodes at the end of its life, molecules can be formed, which are blown into space. In the Interstellar Medium (ISM), in between the stars, the formation of carbon based molecules continues to evolve, leading to complex organic molecules such as Poly Aromatic Hydrocarbons (PAHs) and allotropes, see figure 1. These PAHs are estimated to contain 50 to 150 carbon atoms [1]. Up to 15 % of the carbon in space is locked in PAHs. They are therefore a key player in the interstellar carbon chemistry. This interstellar carbon chemistry is the basis of star and planet formation.
Satellite data
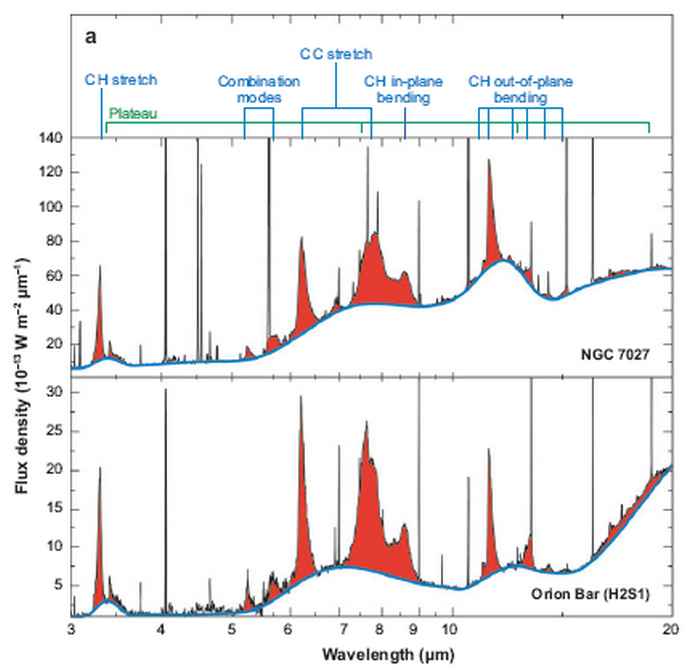
The spectral resolution of the satellite data is not sufficient to resolve all sub-structures in the AIBs. With the upcoming launch of the James Web Space Telescope (JWST) [4], subtle spectral and spatial variations over nebulae will be observable. Experimental laboratory data is required to be able to interpret the variations seen in observations, see figure 3. The available laboratory data is focussed on relatively small PAHs which do not represent interstellar PAHs. The matrix isolation spectroscopy often used introduces small shifts in the peak positions of the same order of these variations [5]. Gas phase spectroscopy of cold, isolated, large molecules is required to extract the molecule specific information of the AIBs.
Chemical evolution
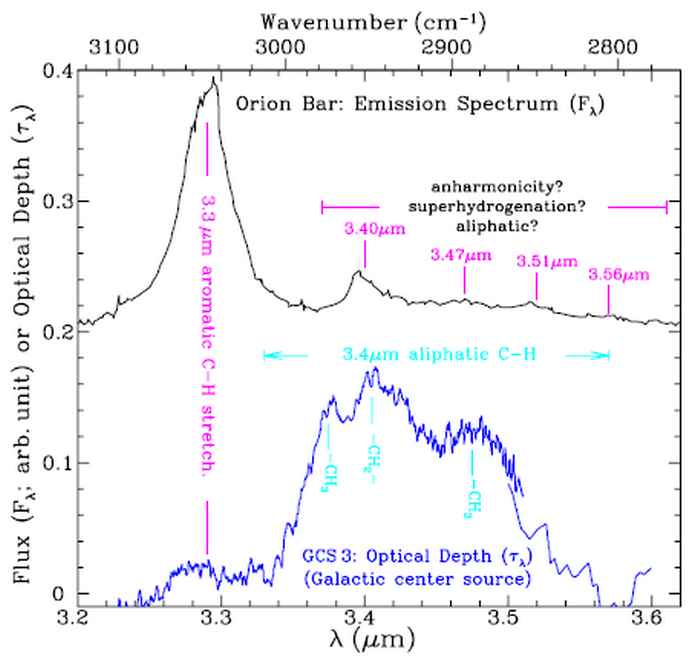
Open questions are: What are the exact shapes, charge, temperature and sizes of the PAHs? What is the chemical evolution of specifically of large PAHs? What are their precursors and their products? [6,7]. The peak profile of the 3.3 mm band gives information about the structure and size of the PAHs. The small features observed to the right of the aromatic C-H stretch band represent aliphatic molecular features that indicate the presence of decorated PAHs, see figure 3.
Our Research
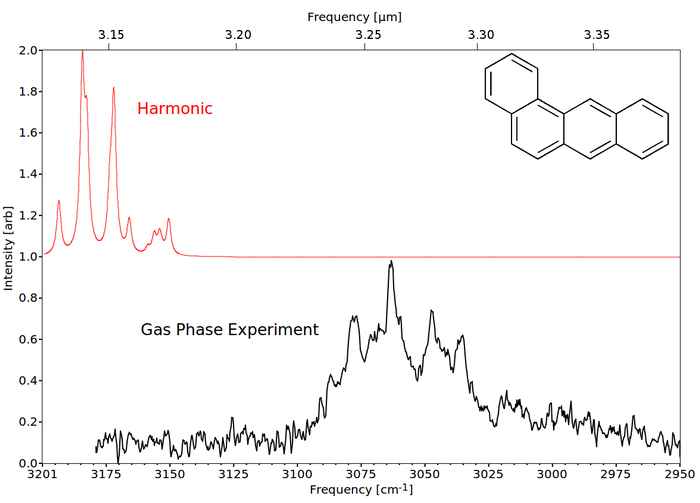
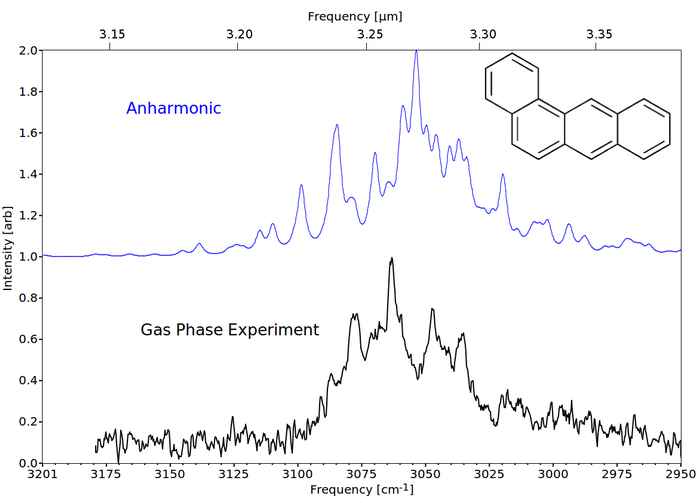
Recently, we have measured the spectra of gas-phase PAHs and decorated PAHs with high resolution and high accuracy up to 5 rings [10-13]. The predicted infrared spectra of these small PAHs employing the standard harmonic approximation do not resemble the experimental laboratory spectra. Inclusion of anharmonicity is required to obtain a good agreement with the experimental spectrum, see figure 4.
We are now focussing on the large PAHs, moving to 50 C-atoms and more. For neutral PAHs and allotropes, we employ laser desorption combined with UV-IR spectroscopy to measure the accurate high-resolution gas-phase spectra of cold astronomically sized PAHs. We aim to determine structure and/or molecule specific spectral behaviour in order to interpret the spectral variations observed in the AIBs that indicate variations the PAH population.
Moreover, we are looking for a link between large PAHs and the UV/visible absorption lines observed in space, the so-called Diffuse Interstellar Bands (DIBs), by means of fluorescence spectroscopy. For the ionic counterparts we employ electrospray ionisation (ESI) / atmospheric pressure photoionization (APPI) and infrared multiple photon dissociation (IRMPD) spectroscopy to measure the gas phase infrared spectra of cations and investigate their breakdown chemistry.

Laboratory work
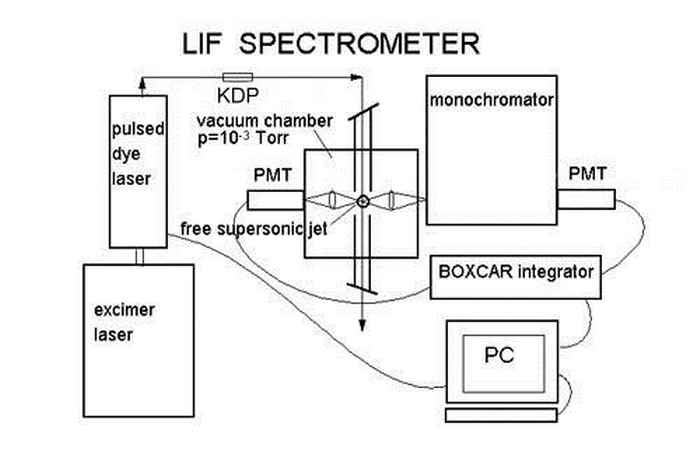
In our laboratory at the UvA, HIMS we use excimer and dye lasers to produce tuneable light in the visible range. The large PAH molecules are brought in the vacuum by means of laser desorption after which they are cooled to several tens of Kelvins using a pulsed gas nozzle. The molecules are excited with the help of the tuneable dye laser and the fluorescence is detected with the help of a photo multiplier tube, see figure 5.
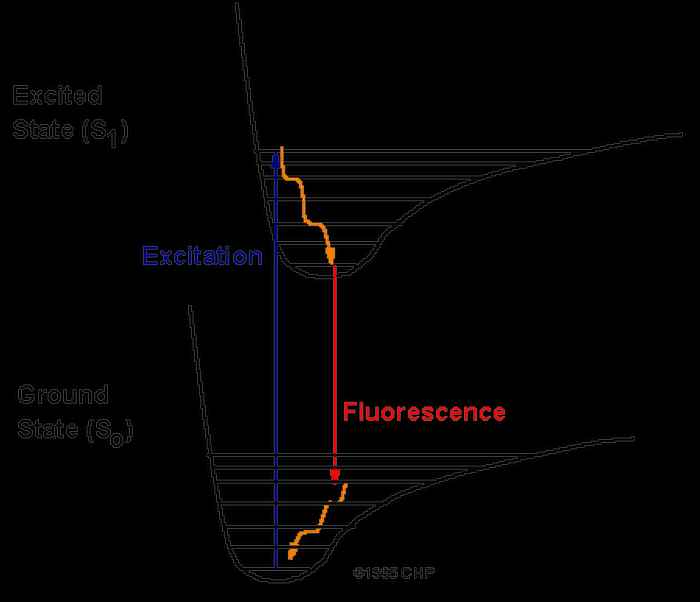
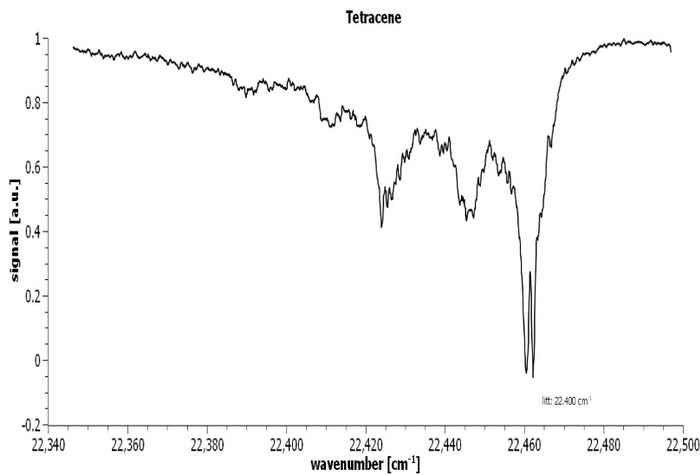
The Aromatic Infrared Bands (AIBs) observed in space originate from fluorescence between vibrational levels of the PAHs, depicted in orange, see figure 6. The experimentally observed fluorescence signal (red arrow) can be compared to the Diffuse Interstellar Bands (DIBS). The vibrational levels of the ground state of the PAHs can be recorded employing IR-UV spectroscopy, see figure 7.
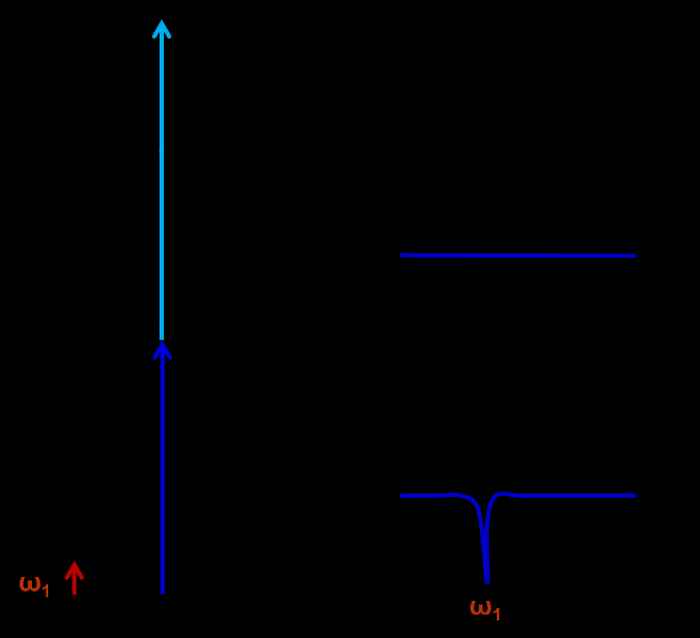
The PAH molecules are ionized employing Resonance Enhanced Multi Photon Ionization (REMPI). A constant mass-selected ion signal is created via a two-color REMPI scheme using a Nd:YAG pumped frequency-doubled dye laser to excite the molecules to the first excited state (S1) and then an ArF excimer laser to ionize the excited molecules. The IR absorption spectra are recorded using an IR–UV depletion scheme. The IR light in the 3-μm region produced by a Nd:YAG pumped frequency-mixed dye laser preceded these two laser beams by 200ns, leading to dips in the ion signal upon IR absorption, see figure 7.
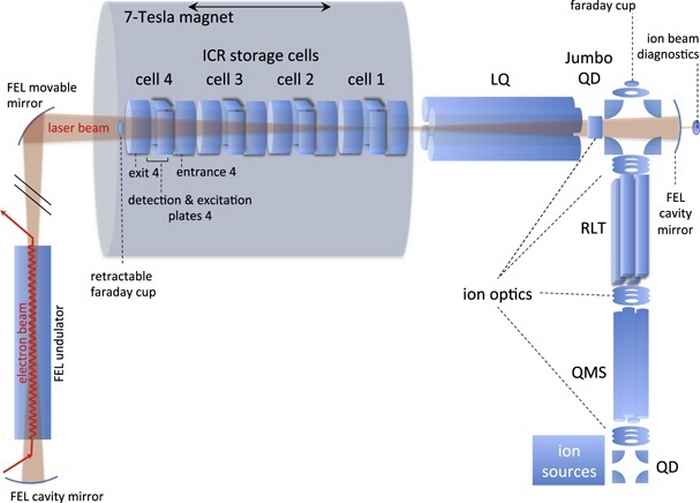
The IR spectra of radical cation PAHs are found to deviate substantially from those of their neutral counterparts. The laboratory study of the IR spectra of ionized PAHs is therefore of profound interest. The FELIX Laboratory at Radboud University (Nijmegen, the Netherlands) exploits intense, short-pulsed infrared and THz free electron lasers [14]. We use a Fourier Transform Ion Cyclotron Resonance Mass Spectrometer (FTICR MS), see figure 8, that is located in the cavity of the Free Electron Laser for Intra-Cavity Experiments, FELICE. The spectroscopic technique applied is IR multiple-photon dissociation (IRMPD), where multiple IR photons are absorbed by mass-isolated PAHs or other cations leading to their dissociation. The intra-cavity arrangement of the FELICE allows for much higher intensities to be attained and promises the ability to record IRMPD spectra of large, highly stable PAHs in isolated, gas-phase conditions [15].
Literature
[1] THE INFRARED SPECTRA OF VERY LARGE, COMPACT, HIGHLY SYMMETRIC, POLYCYCLIC AROMATIC HYDROCARBONS (PAHs): The Astrophysical Journal, 678:316Y327, 2008, C.W. Bauschlicher, Jr., E. Peeters and L.J. Allamandola.
[2] Astronomical observations of the PAH emission bands: Peeters, E. 2011, EAS Publications Series, Volume 46, 13 (http://www.eas-journal.org/)
[3] The molecular universe, Rev. Modern Phys. 85 (2013), A.G.G.M. Tielens.
[4] http://www.jwst.nasa.gov/
[5] http://www.astrolab.uni-jena.de/index.php/projects-and-interests?id=10
[6] LABORATORY FORMATION OF FULLERENES FROM PAHS: TOP-DOWN INTERSTELLAR CHEMISTRY: The Astrophysical Journal Letters, 797:L30 (5pp), 2014, J. Zhen, P. Castellanos, D.M. Paardekooper, H. Linnartz, and A.G.G.M. Tielens.
[7] Top-down formation of fullerenes in the interstellar medium: A&A, 577 (2015) A133, O. Berné1, J. Montillaud and C. Joblin. http://dx.doi.org/10.1051/0004-6361/201425338
[8] THE STRUCTURE, ORIGIN, AND EVOLUTION OF INTERSTELLAR HYDROCARBON GRAINS: The Astrophysical Journal, 770:78 (13pp), 2013. J. E. Chiar1, A. G. G. M. Tielens2, A. J. Adamson and A. Ricca.
[9] THE CARRIERS OF THE INTERSTELLAR UNIDENTIFIED INFRARED EMISSION FEATURES: AROMATIC OR ALIPHATIC?: The Astrophysical Journal Letters, 760:L35 (5pp), 2012. A. Li and B. T. Draine.
[10] The anharmonic quartic force field infrared spectra of three polycyclic aromatic hydrocarbons: Naphthalene, anthracene, and tetracene: The Journal of Chemical Physics 143, 224314 (2015); doi: 10.1063/1.4936779, Cameron J. Mackie, Alessandra Candian, Xinchuan Huang, Elena Maltseva, Annemieke Petrignani, Jos Oomens, Wybren Jan Buma, Timothy J. Lee, and Alexander G. G. M. Tielens.
[11] High-resolution IR absorption spectroscopy of polycyclic aromatic hydrocarbons: the realm of anharmonicity: The Astrophysical Journal, 814:23 (2015); doi:10.1088/0004-637X/814/1/23, Elena Maltseva, Annemieke Petrignani, Alessandra Candian, Cameron J. Mackie, Xinchuan Huang, Timothy J. Lee, Alexander G. G. M. Tielens, Jos Oomens, and Wybren Jan Buma.
[12] High-resolution IR absorption spectroscopy of polycyclic aromatic hydrocarbons in the 3-m region: Role of periphery; to be published, Elena Maltseva, Annemieke Petrignani, Alessandra Candian, Cameron J. Mackie, Xinchuan Huang, Timothy J. Lee, Alexander G. G. M. Tielens, Jos Oomens and Wybren Jan Buma.
[13] The anharmonic quartic force field infrared spectra of five non-linearPolycyclic Aromatic Hydrocarbons: benz[a]anthracene, chrysene, phenanthrene, pyrene, and triphenylene; to be published; Cameron J. Mackie, Alessandra Candian, Xinchuan Huang, Elena Maltseva, Annemieke Petrignani, Jos Oomens, Andrew L. Mattioda, Wybren Jan Buma, Timothy J. Lee, and Alexander G. G. M. Tielens
[14] http://www.ru.nl/felix/facility-0/measurement-station/fticr-mass/
[15] Breadown products of gaseous polycyclic aromatic hydrocarbons investigated with infrared ion spectroscopy: The Astrophysical Journal, Volume 826, Number , A. Petrignani, M. Vala, J. R. Eyler, A. G. G. M. Tielens, G. Berden, A. F. G. van der Meer, B. Redlich, and J. Oomens.