Towards pericyclic organophosphorus chemistry
29 January 2018
In organic chemistry, the use of pericyclic reactions is a powerful, atom-economical tool that provides access to strained molecular ring systems with interesting topology. Cyclopentadienone, tricyclopentanone, and housene are a few striking examples.
The incorporation of heteroatoms such as phosphorus into these molecules is an appealing concept, which provides a coordination site thereby creating a novel class of phosphine ligands with different electronic and steric properties.
Phosphorus analogues

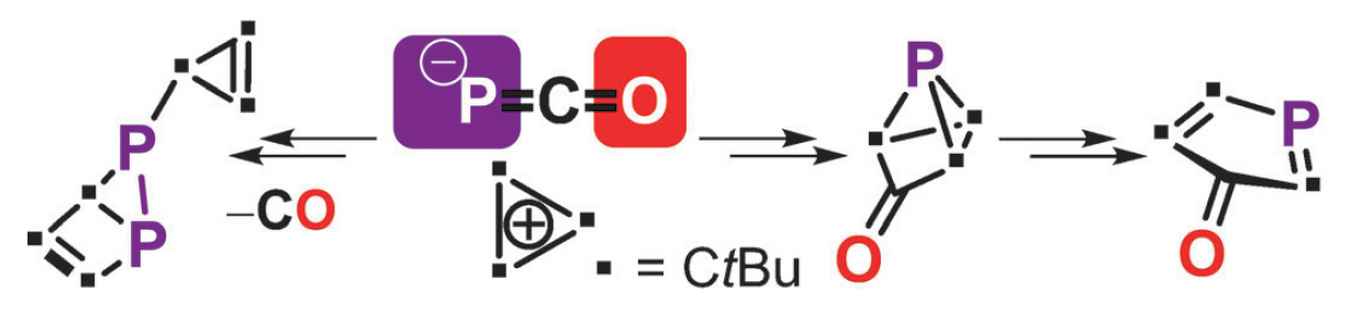
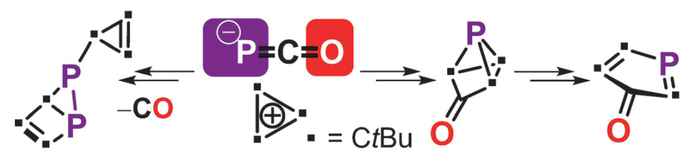
The Amsterdam researchers, together with chemists from the universities of Johannesburg, Helsinki and Utrecht, now report in Angewandte Chemie the synthesis of the phosphorus analogues of cyclopentadienone, tricyclopentanone, and housene. Starting with 1,2,3-tris-tert-butylcyclopropenium tetrafluoroborate and using sodium phosphaethynolate as the phosphorus-providing moiety they were able to synthesize cyclopropenylphosphaketene as a starting material to explore the proposed pericyclic organophosphorus chemistry.
In a thorough study, they combined perseverance in synthetic chemistry with determination of molecular structures using advanced NMR and X-ray techniques, and were able to provide detailed mechanistic analysis supported by DFT calculations. They thus show that cyclopropenylphosphaketene and its dimer 1,3-diphosphetane-2,4-dione grant access to the phosphorus analogues of housene, tricyclopentanone, and cyclopentadienone, all of which display intriguing pericyclic reactions.
Knowledge on key concepts
In their current research, the Amsterdam chemists are developing decarbonylation strategies for phosphatricyclopentanone. They hope this will ultimately lead to the synthesis of phosphacyclobutadiene and phosphatetrahedrane, molecules that are the phosphorus analogues of the archetypical anti-aromatic cyclobutadiene and the highly strained tetrahedrane.
This would not only be a great synthetic achievement; these molecules will also provide knowledge on key physical organic chemistry concepts on molecular structure and bonding, such as aromaticity, ring strain, and thermal and photochemical pericyclic reactions.
The research was carried out as part of the VIDI research of Dr Chris Slootweg, funded by the Netherlands Organisation for Scientific Research (NWO). It was also supported by the European Union through a Marie Curie grant for the SusPhos International Training Network.
Article
T. Krachko, A. W. Ehlers, M. Nieger, M. Lutz, J. C. Slootweg: Synthesis and Reactivity of the Phosphorus Analogues of Cyclopentadienone, Tricyclopentanone, and Housene, Angew. Chem. Int. Ed. 2018, 57, 1683. DOI: 10.1002/anie.201711838