Supramolecular nanoconcentrators boost performance of water oxidation catalyst
12 July 2018
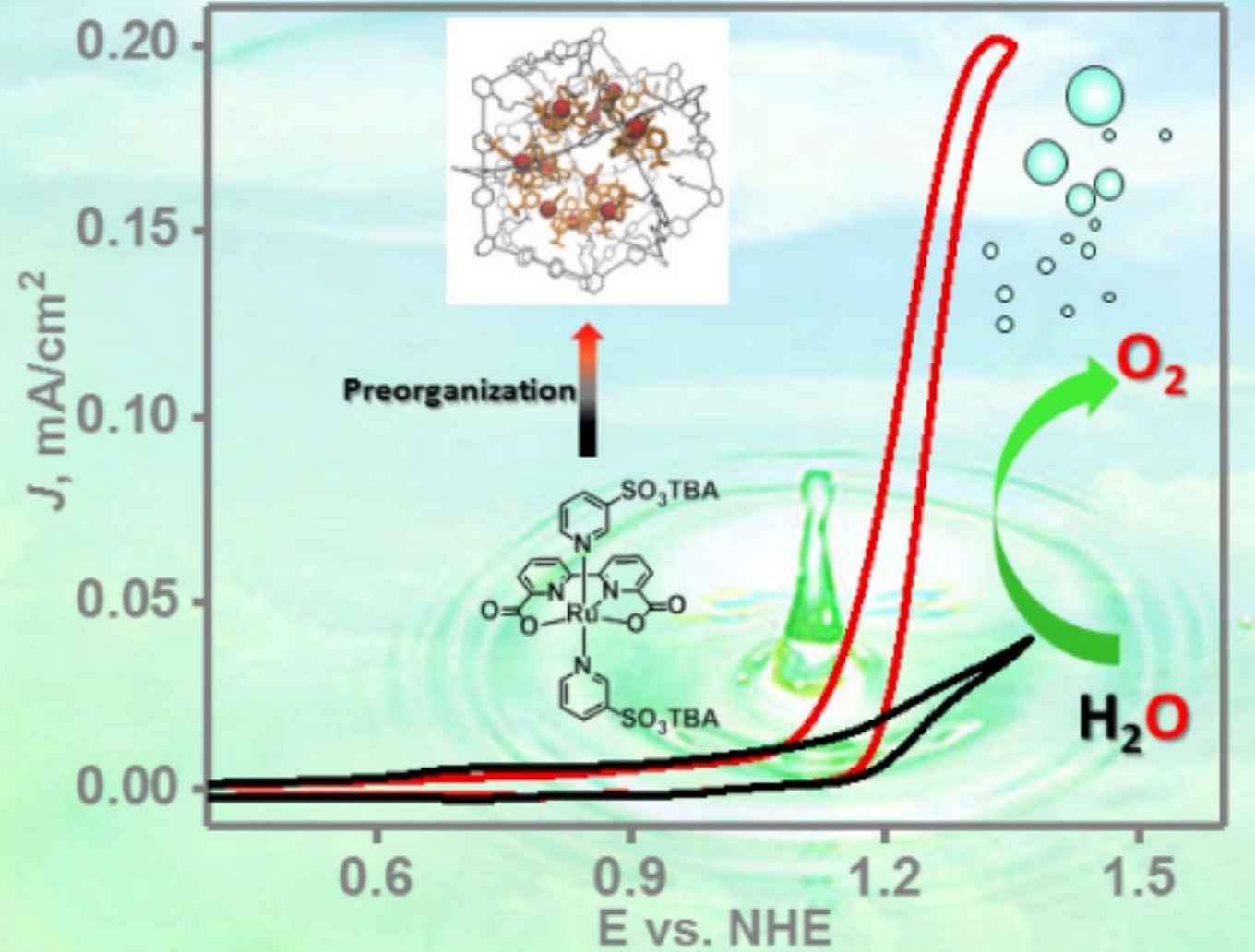
Renewable fuel generation is of crucial importance for the energy transition required to meet the climate goals for 2030 and 2050. For the generation of such solar fuels, by artificial photosynthesis or via power to fuel approaches, the electrochemical splitting of water to obtain oxygen and hydrogen is a crucial step. To do this efficiently, suitable catalysts that enable rapid catalysis at low overpotentials are required.
In the past decades, chemists have developed molecular water oxidation catalysts based on various transition metals (Ru, Ir, Cu, Fe, and Ni). In particular ruthenium-based catalysts perform very well in terms of activity, low overpotential and stability. However, they lose their excellent performance under the typical dilute catalyst concentrations used for electrocatalytic processes.
Nanoconcentrator
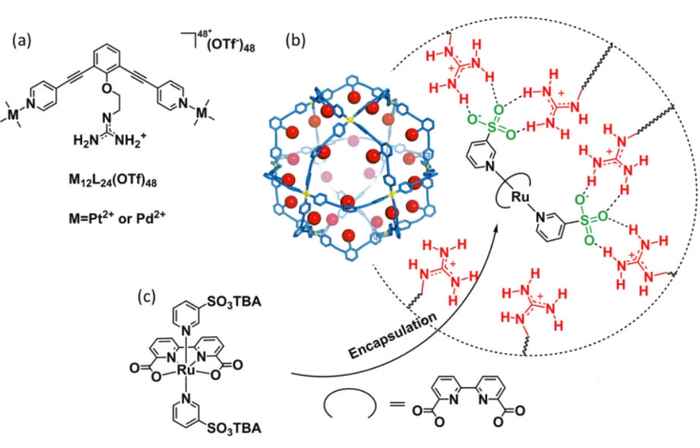
To be able to use the full potential of the ruthenium water splitting catalysts under these conditions, the Amsterdam researchers have now applied their concept of a 'nanoconcentrator'. It was inspired by the working principles of natural enzymes that bind molecules in well-defined pockets close to their active sites, facilitating highly efficient transformations by pre-organisation of crucial components. The Reek group mimics this enzymatic behaviour with synthetic self-assembling nanospheres, functionalized with guanidine binding sites to accommodate multiple active catalyst centers per nanosphere.
In their publication, which has just been accepted by Angewandte Chemie International Edition, the researchers present a modified ruthenium water splitting catalyst complex capable of binding to the guanidine sites. Their research shows that up to twelve catalyst complexes can be accommodated per nanosphere, thus enhancing the concentration of a diluted solution of 2.5 × 10-5 M to a local concentration of 0.54 M. This results in a two-orders of magnitude increase in the rate of electrochemical water oxidation by the ruthenium catalyst.
Reaction mechanism
By combining experimental and spectroscopic data with molecular dynamics simulations, the researchers were able to elucidate the reaction mechanism of the catalyzed water splitting in their nanospheres. They conclude it includes the coupling of two metal-oxyl radicals (a so-called I2M mechanism) and show that at very high local concentrations the reaction rate is no longer dependent on this radical coupling step, thus effectively eliminating the diffusion limitations that occur at very low overall catalyst concentrations.
Furthermore, since solubility limitations and other practical issues would not allow to perform electrocatalysis in the higher concentration regime, their supramolecular nanoconcentrator strategy represents an important new tool to maximize catalyst efficiency by controlling the reaction mechanism. It also provide new tools to study the mechanism of electrocatalytic conversions.
The research is part of UvA's research priority area Sustainable Chemistry and was funded through Joost Reek's ERC Advanced Grant in 'Nature Inspired Transition Metal Catalysis'.
Article
Fengshou Yu, David Poole III, Simon Mathew, Ning Yan, Joeri Hessels, Nicole Orth, Ivana Ivanović-Burmazović and Joost N. H. Reek: Control over Electrochemical Water Oxidation Catalysis by Preorganization of Molecular Ruthenium Catalysts in Self‐Assembled Nanospheres. Angewandte Chemie International Edition, accepted. DOI:10.1002/anie.201805244