Francesco Mutti listed among research talents by journal ChemBioChem
26 March 2019

The peer-reviewed journal of chemical biology, synthetic biology and bio-nanotechnology maintains a special online issue entitled ChemBioTalents containing selected papers of the talented researchers. ChemBioTalents thus showcases the next generation of emerging scientists which are nominated by ChemBioChem board members, with a few additional selections from the editorial office. The selected researchers are considered 'to be key figures that will shape the future of research at the interface of chemistry and biology'.
Promiscuous amine dehydrogenases
Dr. Mutti contributes to ChemBioTalents with an article on 'Mechanistic Insight into the Catalytic Promiscuity of Amine Dehydrogenases: Asymmetric Synthesis of Secondary and Primary Amines'.
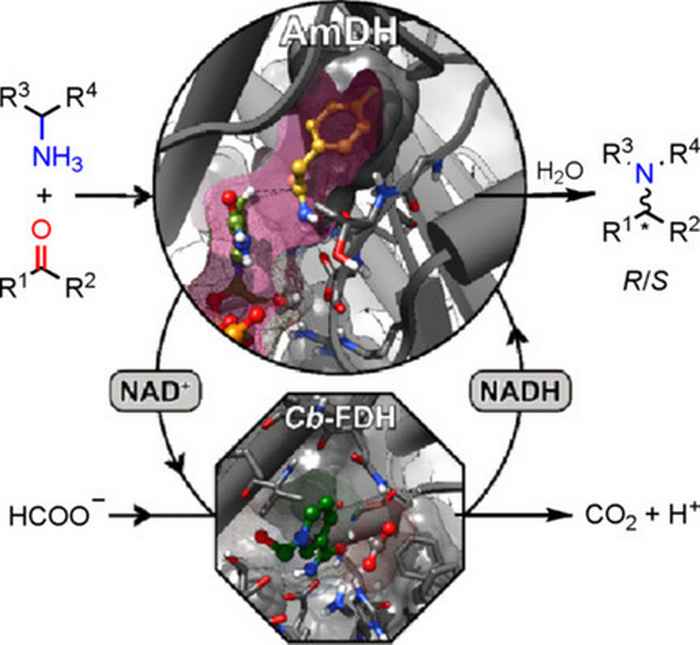
The paper recently also appeared in the regular printed edition of ChemBioChem and describes research performed with his co-workers Vasilis Tseliou, Marcelo Masman, Wesley Böhmer and Tanja Knaus. The team shows for the first time that amine dehydrogenases are capable of accepting alternative amine donors than ammonia, thus yielding enantioenriched secondary amines.
Their paper also reports the promiscuous formation of enantiopure primary amines along with the expected secondary amines. This is a surprising discovery since ammonia was not present—as most obvious amine donor—in the reaction media. Combining practical laboratory experiments with computational experiments, the research team explained these findings through an unprecedented reaction mechanism that entails the isomerization of the iminium intermediate formed in the catalytic cycle. Notably, this is the first report of a formal transamination that does not involve the natural cofactor pyridoxal-phosphate, but uses nicotinamide adenine dinucleotide.
Francesco Mutti obtained his Master's degree in industrial chemistry summa cum laude (2004) and his Ph.D. in chemistry (2008) from the University of Milan. After post‐doctoral work in the groups of Prof. Wolfgang Kroutil at the University of Graz (2009–2012) and Prof. Nicholas Turner at The University of Manchester (2013–2014), he moved to the University of Amsterdam (2015). He has published more than 40 research papers, several book chapters and 5 patents. His research interests are in the area of biocatalysis—developing biocatalytic cascades for the sustainable manufacture of chemical products—enzyme discovery, and enzyme engineering.
Publication details
V. Tseliou, M. F. Masman, W. Böhmer, T. Knaus and F. G. Mutti: 'Mechanistic Insight into the Catalytic Promiscuity of Amine Dehydrogenases: Asymmetric Synthesis of Secondary and Primary Amines'. ChemBioChem 2019 Mar 15; 20(6):800-812; DOI: 10.1002/cbic.201800626