The use of dioxazolones for the catalytic transfer of acyl nitrenes
Review in ACS Catalysis by HIMS PhD student Kaj van Vliet
23 April 2020
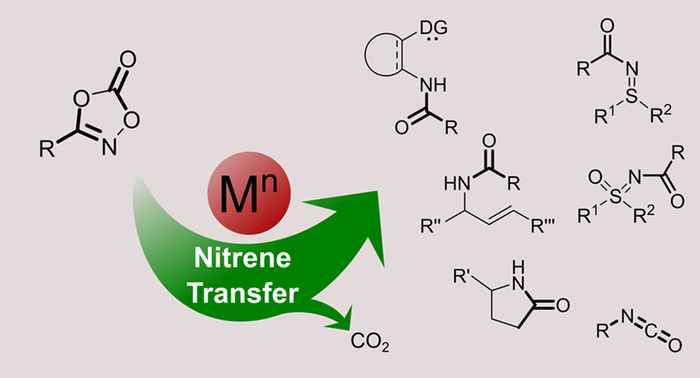
The ease of dioxazolone synthesis, formation of CO2 gas as the sole byproduct from reactions with dioxazolones, and the general importance of nitrene transfer reactions will undoubtedly lead to more interesting reactions in the near future. Given the general importance of nitrene transfer reactions, recently a range of interesting reactions producing N-aryl amides, oxazoles, and lactams was published. Furthermore, since the activation of dioxazolones proceeds under mild reaction conditions, stereo- and enantioselective reactions are also possible, which is useful for the synthesis of bioactive nitrogen-containing compounds.
Last year, in the Journal of the American Chemical Society, Kaj van Vliet published a fast and practical copper-catalyzed reaction of aryl acetylenes, amines, and easily accessible 1,4,2-dioxazol-5-ones to N-acyl amidines. Now, in ACS Catalysis, he provides an overview of all relevant dioxazolone reactions reported in recent years.
Van Vliet reports that dioxazolones are typically activated by transition metals at relatively low reaction temperatures. The metal nitrenoids formed by decarboxylative activation of dioxazolones are generally electron-deficient and commonly react in a concerted fashion. “Intermolecular” nitrene insertion reactions involving preactivated C–H bonds (“inner-sphere” mechanism) easily compete with the Curtius-type rearrangement, but for intramolecular “direct” nitrene transfer/insertion reactions involving nonpreactivated substrates (i.e., without preceding formation of metal–carbon or metal–hydride bonds) extensive ligand optimization is important to prevent such unwanted side reactions.
Publication details
Kaj M. van Vliet, Bas de Bruin: Dioxazolones: Stable Substrates for the Catalytic Transfer of Acyl Nitrenes. ACS Catal. 2020, 10, 8, 4751-4769 DOI: 10.1021/acscatal.0c00961
See also
Kaj M. van Vliet, Lara H. Polak, Maxime A. Siegler, Jarl Ivar van der Vlugt, Célia Fonseca Guerra, Bas de Bruin: Efficient Copper-Catalyzed Multicomponent Synthesis of N-Acyl Amidines via Acyl Nitrenes. J. Am. Chem. Soc. 2019, 141, 38, 15240-15249 DOI: 10.1021/jacs.9b07140