Redox-mediated alcohol oxidation coupled to hydrogen formation in a dye-sensitized photosynthesis cell
22 September 2020
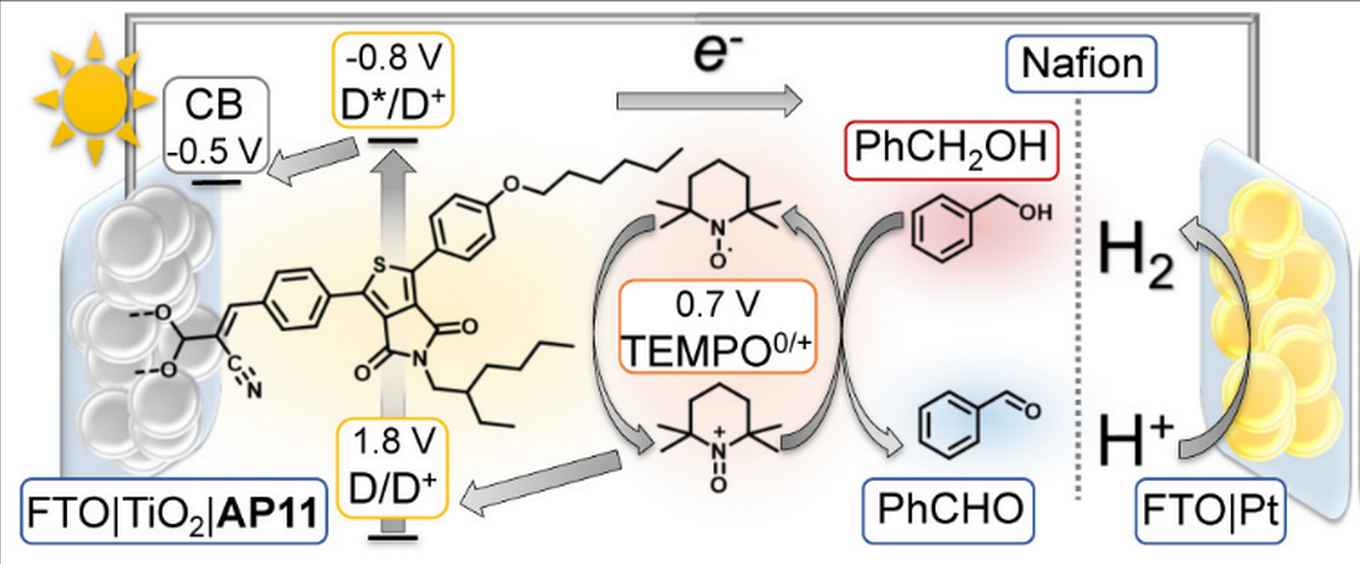
Usually, these types of dye sensitised cells are designed for water oxidation, where the light-driven production of fuel (hydrogen) is combined with oxygen formation. Researchers at the University of Amsterdam's Van 't Hoff Institute for Molecular Sciences now demonstrate that fuel production can be combined with a relevant chemical oxidation reaction. Crucial for the device was the dual role for the TEMPO molecule, which serves both as redox mediator and as a catalyst for the oxidation reaction. This leads to higher efficiencies and quantitative Faradaic efficiency. A new reactor was designed that separates the two reactions in separate compartments and enables in situ reaction monitoring of substrates and products. This is important for extending application of the cell to other organic reactions.
Abstract
We report a dye-sensitized photoelectrochemical cell (DSPEC) that couples redox-mediated light-driven oxidative organic transformations to reductive hydrogen (H2) formation. The DSPEC photoanode consists of a mesoporous anatase TiO2 film on FTO (fluorine-doped tin oxide), sensitized with the thienopyrroledionebased dye AP11, while H2 was formed at a FTO–Pt cathode. Irradiation of the dye-sensitized photoanode transforms 2,2,6,6- tetramethylpiperidine 1-oxyl (TEMPO) to the oxidized TEMPO (TEMPO+), which acts as a chemical oxidant for the conversion of benzyl alcohol. The TEMPO0/+ couple, previously used as redox mediator in DSSC, mediates efficient electron transfer from the organic substrate to the photo-oxidized dye. A DSPEC photoreactor was designed that allows in situ monitoring the reaction progress by infrared spectroscopy and gas chromatography. Sustained light-driven oxidation of benzyl alcohol to benzaldehyde within the DSPEC photoreactor, using of TEMPO as a mediator, demonstrated the efficiency of the device, with a photocurrent of 0.4 mA cm-2, approaching quantitative Faradaic efficiency and exhibiting excellent device stability.
Paper
Didjay F. Bruggeman, Tijmen M. A. Bakker, Simon Mathew and Joost Reek: Redox‐Mediated Alcohol Oxidation Coupled to Hydrogen Gas Formation in a Dye‐Sensitized Photosynthesis Cell. Chem.Eur.J, First published 09 September 2020. DOI: 10.1002/chem.202003306