Convenient methodology to forge C‒N bonds
8 June 2021
The research is part of the HAT-TRICK project of postdoc researcher Luca Capaldo for which he received a Marie Curie Fellowship earlier this year. It revolves around Hydrogen Atom Transfer (HAT) in an approach that merges photocatalysis, electrochemistry and flow chemistry.
Abstract
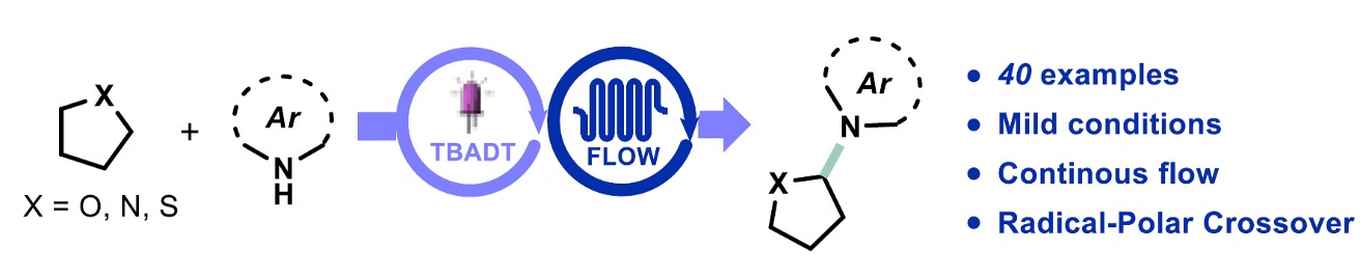
Photocatalytic hydrogen atom transfer is a very powerful strategy for the regioselective C(sp3)‒H functionalization of organic molecules. Herein, we report on the unprecedented combination of decatungstate hydrogen atom transfer photocatalysis with the oxidative Radical-Polar Crossover concept to access the direct netoxidative C(sp3)‒H heteroarylation. The present methodology demonstrates a high functional group tolerance (40 examples) and is scalable when using continuous-flow reactor technology. The developed protocol is also amenable to the late-stage functionalization of biologically relevant molecules such as stanozolol, (‒)-ambroxide, podophyllotoxin and dideoxyribose.
Paper
Ting Wan, Luca Capaldo, Gabriele Laudadio, Alexander Nyuchev, Juan Rincon, Pablo Garcia-Losada, Carlos Mateos Gutierrez, Michael O. Frederick, Manuel Nuno, and Timothy Noel: Decatungstate-mediated C(sp3)‒H Heteroarylation via Radical-Polar Crossover in Batch and Flow. Angew. Chem. Int. just accepted. DOI: 10.1002/anie.202104682