Unravelling the secrets of a fungal 'raincoat'
14 April 2017
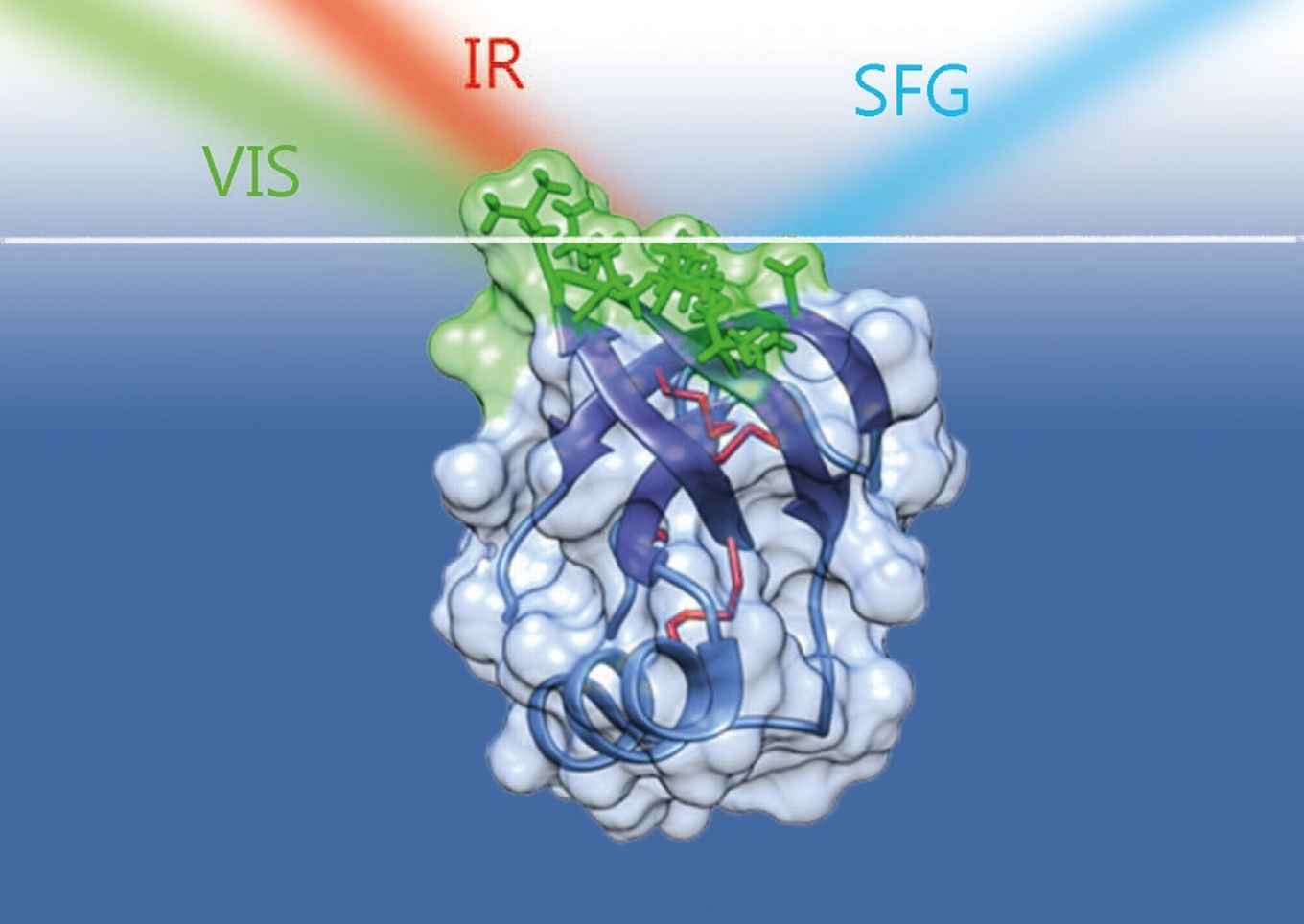
The research was performed by researchers at AMOLF together with colleagues from the Molecular Photonics research group at the University of Amsterdam's Van 't Hoff Institute for Molecular Sciences (HIMS) and researchers from Finland. Their results have recently been published in the Journal of Physical Chemistry Letters.
Natural rain coat
Hydrophobins are small surface proteins that are expressed only by filamentous fungi ('moulds') to form a protective film that act as a kind of natural 'rain coat'. The films are very stable, show an exceptionally large elasticity and are highly water-repellent, ensuring that the fungi can cope with many different environments.
How individual hydrophobins molecules rivet together (self-assemble) and create firm elastic surfaces is not yet well understood. The Amsterdam researchers contribute to a better understanding by their detailed study using vibrational sum-frequency generation spectroscopy, a highly surface-sensitive technique which is ideal to probe the molecular conformation and orientation at interfaces.
Acidity-induced reorientation
By means of measurements performed at AMOLF (postdoc Konrad Meister) and spectroscopic calculations performed at HIMS (PhD student Steven Roeters and professor Sander Woutersen) a strong relation could be established between the orientation of the individual hydrophobin molecules and the acidity of the water on which the hydrophobin film is floating. As the water phase becomes more acidic, individual proteins become more tilted and this acidity-induced reorientation decreases the elasticity of the surface film. This finding can lead to a new method to create hydrophobin films with desired visco-elastic properties, ultimately leading to the creation of novel designer microfilms of industrial relevance.
Reference
Konrad Meister, Steven J. Roeters, Arja Paananen, Sander Woutersen, Jan Versluis, Géza R. Szilvay, and Huib J. Bakker, Observation of Ph-induced protein reorientation at the water surface, Journal of Physical Chemistry Letters, DOI: 10.1021/acs.jpclett.7b00394