Left or right? Novel algorithm takes chirality determination to the next level
Results published as 'hot paper' and 'pick of the week' in RSC journal Chemical Science
30 August 2019
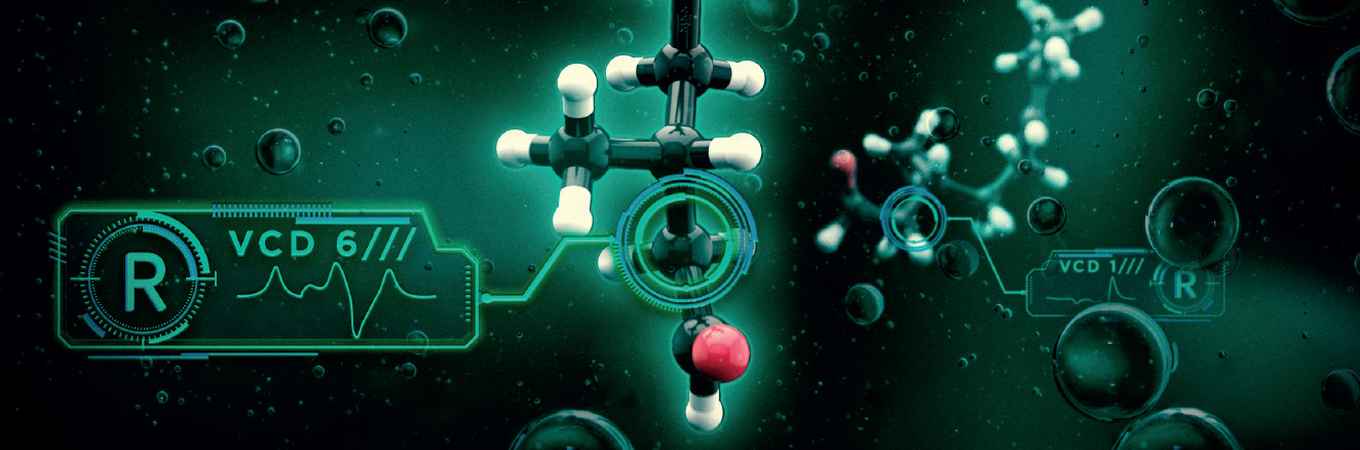
The team led by professor Wybren Jan Buma publish their novel VCD method in the 7 September issue of Chemical Science, the flagship journal of the Royal Society of Chemistry. In recognition of its importance, the paper features on the cover and was designated as a 'hot paper' and 'pick of the week' by the ChemScience editors.
According to first author PhD student Mark Koenis 'it is now possible to determine the handedness of molecules much more reliably and with better quantitative measures than before'.
In their paper, Buma and co-workers demonstrate their novel approach, amongst others, by studies on citronellal. It is a typical example of the class of molecules that have until now posed challenges - often insurmountable - to VCD analysis. It is chiral, meaning that it can exist as two molecular structures that are non-superimposable mirror images of each other - just like a right hand and a left hand. It is also a very flexible and dynamic molecule that can adopt many different spatial structures, called conformations.
Spatial variation confounds chirality determination
Being chiral, citronellal represents a class of molecules of great biochemical and pharmaceutical relevance. Since many biological molecules (proteins, enzymes, receptors, and so forth) are chiral, the 'handedness' of chiral molecules determines their biological interactions. In the case of citronellal, its chiral mirror structures (called enantiomers) differ in interaction with olfactory receptors so that the 'left-handed' molecule smells of oranges and its 'right-handed' counterpart of lemons. In many other molecules, the effect of chirality can be much more dramatic. In pharmaceutical applications, for example, one enantiomer of a drug may have a beneficial therapeutic effect, while the other has harmful biological consequences.

Being flexible and dynamic, citronellal illustrates the challenges of chirality determination by means of VCD spectroscopy. VCD makes use of circularly polarized light that in fact displays a 'handedness' in the difference between the left and right circular polarization. Thus, it enables to distinguish between left- and right-handed molecules. The sophisticated technique yields a spectroscopic fingerprint that is unique for each molecule and even for each mirror image of the same molecule. In fact, for all practical purposes, VCD is the only technique capable of distinguishing between enantiomers under real-life conditions.
The snag, however, is that, just like citronellal, many molecules are flexible and dynamic, adopting many different spatial structures. Each structure has its own fingerprint so that an actual VCD spectrum is the total of all fingerprints of all spatial molecular variants present in the sample. Adding to this, more stable, low-energy variants will be more present than higher-energy ones so that not all variants contribute equally to the VCD spectrum. The structural freedom thus forms a serious problem for determining chirality in these cases.
Genetic algorithm
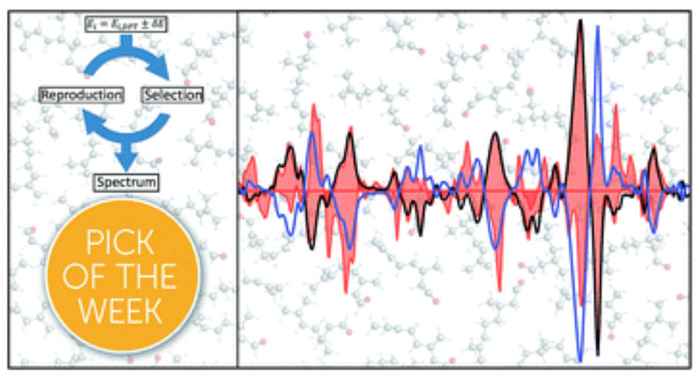
The customary solution in VCD analysis is to determine all possible conformations of the molecule under investigation, calculate their energies and corresponding fingerprints, and then average these individual components and compare the resulting spectrum with the experimental VCD spectrum.

This is, however, much less clear-cut then it might appear. Many methods are available for calculation of energies of the various spatial structures, from very simple to very advanced. According to Buma, 'in the worst case it might be that one type of calculation would lead to the conclusion that the molecule has one particular type of handedness, while another type of calculation would lead to the opposite conclusion'.
His team has now significantly improved the 'calculate and compare' strategy by explicitly taking the uncertainty in the calculated energies into account. Using a genetic algorithm which uses the principles of evolution and “survival of the fittest” they could adjust the contributions of the various fingerprints in such a way that the best agreement with the experimental VCD spectrum was obtained. 'The beauty of our approach is that the correct handedness always leads to better agreement with the experimental data than the opposite handedness', says Koenis. 'Even more importantly, it enables us to present a quantitative measure of the reliability of the VCD assignment.'
Increasing opportunities for application
The genetic algorithm was not only tested on citronellal but also on dehydroquinidine, a chiral molecule representing a ‘worst-case’ scenario because it shows large dynamic structural changes.
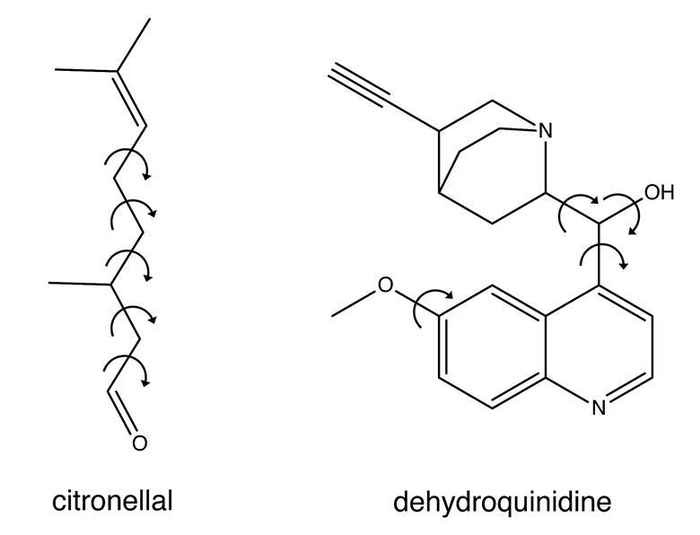
Moreover, the VCD spectrum of dehydroquinidine is experimentally much harder to obtain and the available spectrum is therefore of a much lower quality than what is normally aimed for. The results show that even for such 'difficult' molecules the novel approach is by far superior to all existing methods for absolute configuration assignment.
The researchers expect that their improvement of the reliability of VCD as an analytical tool will bring applications within reach such as quality control in the production of pharmaceutical ingredients. They have already performed studies to determine levels of chiral impurities using VCD. 'We have also shown that notoriously difficult problems such as molecules with many chiral centres can be tackled', says Buma. Taken into consideration that VCD is experimentally more simple and cost-effective than other techniques, he foresees increasing opportunities for application of the technique both in development and large-scale production of chiral molecules.
Publication details

Mark A. J. Koenis, Yiyin Xia, Sérgio R. Domingos, Lucas Visscher, Wybren Jan Buma and Valentin P. Nicu: Taming conformational heterogeneity in and with vibrational circular dichroism spectroscopy Chem. Sci. 2019, 10, 7680-7689 DOI: 10.1039/C9SC02866H
Read more:
ChemSci pick of the week: Exceptional measurement of chirality
Chemistry World news item: Algorithm increases certainty of stereochemical assignments in flexible molecules