NWO-ECHO grant for Jan van Maarseveen
For further development of lasso peptide synthesis
18 December 2017

Van Maarseveen aims to further develop his recently developed 'template-based backfolding concept' into a successful strategy for the synthesis of lasso peptides. Earlier this year his group reported in Nature Communications on the synthesis of pretzel-like molecules as a first important step towards lasso peptides.
Unique class of molecules
Lasso peptides make up a unique class of molecules displaying promising biological activities. Over the last decades many lasso peptides have been isolated from microorganisms. Research has shown they lack much of the undesired properties which render regular peptides unsuitable as drugs. It therefore is highly desirable to synthetically open up the lasso peptide series and explore their pharmaceutical use.
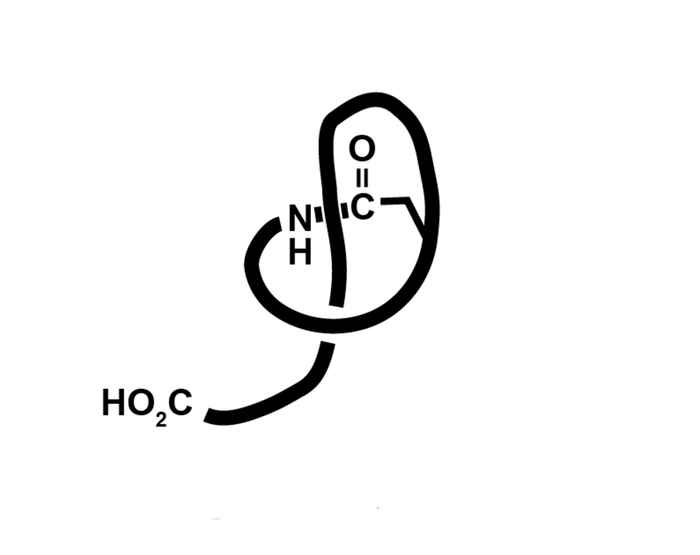
Their synthesis, however, has turned out to be a rather challenging endeavor. Although several research groups have embarked on this subject, the fact that almost fifteen years after their structure elucidation no synthesis has been published underscores their topological complexity. Even the 2016 Nobel prize-winning methodologies toward rotaxanes/catenanes that are mainly based on supramolecular mutual positioning of the ring-thread fragments, fall short.
Closing the loop
In the synthetic concept under development by the UvA Synthetic Organic Chemistry group, the loop of the lasso peptide is forced to close in the right place around the rope. To achieve this a linear ring precursor is covalently positioned in a perpendicular arrangement over the thread fragment, using the 3D geometry of a tetrahedral carbon atom. Subsequently a backfolding cyclization at a specific position is performed. The ECHO project will start with the development of the new methodology toward relatively simple peptide-like [2]rotaxanes, eventually arriving at the natural peptide [1]rotaxanes.
ECHO project grants are 260,000 euro project grants for excellent chemical research. The grants offer the opportunity to carry out a high quality science driven research project. In this way a challenging scientific idea can be pursued, a new research area can be opened up or a scientific innovation can be established.