Development of useful and robust methodology for preparing small cyclic peptides

Cyclic peptide-like molecules are highly desirable starting points for drug development. Cyclizations of linear precursors are usually severely hampered by unfavourable conformations, so that bimolecular reactions successfully compete.
Our main approach to strained lactams aims to preorganize the cyclization precursor in a smart fashion by applying an aromatic template allowing easy cyclization to a larger ring followed by ring contraction and template removal. In this way several 7- and 8-membered lactams were prepared which were unavailable otherwise (H. Bieräugel, T.P. Jansen, CW/NWO-supported).
A novel linker has been developed allowing facile cleavage by treatment with a TFA/TMSOTf cocktail. This linker has also been immobilized onto polystyrene resin in order to allow combinatorial access to strained lactams (J. Springer, CW/NWO-supported, cooperation with Solvay Pharmaceuticals).
Another approach was to the use the shielding effect exerted within the core of a dendrimer to promote cyclization over oligomerization reactions. Dendrimers with a diimide moiety in the core indeed showed higher lactamization efficiencies as compared to small diimides (A. Amore, UvA funded, PhD June 2, 2005).
The copper(I)-catalyzed azide-alkyne “click” cycloaddition was used as the macrocyclization reaction. This so-called click reaction leads to a 1,2,3-triazole which is a close structural model for a transoid amide moiety, thus enhancing the structural variety for biological screening (R. von Eggelkraut-Gottanka, S. Calvet, CW/NWO-supported, cooperation with Organon).
All psi-triazole analogs of c[Pro-Val-Pro-Tyr], a natural cyclic tetrapeptide that is synthetically inaccessible, have been prepared successfully. These analogues showed equipotent or even better tyrosinase inhibition potency than the native peptide underscoring that triazoles indeed are effective trans-amide bond surrogates (collaboration with dr. D. Speijer, AMC).
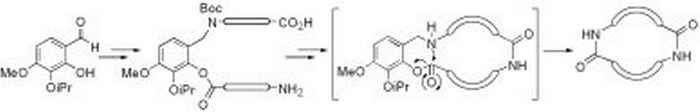
Our intramolecular Staudinger ligation method has been applied for the synthesis of lactams in which a biaryl moiety is part of the ring system. Such systems bear resemblance to the vancomycin antibiotics, where axial chirality plays an important role. Although our method showed very efficient ring-closure, all efforts to transfer phosphorus chirality to axial chirality in the Staudinger ligation process failed (G. Masson, Marie Curie fellow, EC-supported).
In 2006 BMS associate professor Jan van Maarseveen was portrayed in the 'Focus on Research' series on the UvA webpages. Click below to read about his research on cyclic peptides.