Ning Yan selected as 'Emerging Investigator' in Green Chemistry
Sustainable Chemistry tenure-tracker is one of the 'rising stars of the green chemistry field'
9 July 2017

Ning Yan was recognised for his work on bifunctional electrocatalysts that can do both oxygen reduction and oxygen evolution. Together with his team Yan developed a new bifunctional electrocatalyst that can be used in various fuel cell electrodes. They used a facile yet effective approach to prepare unique nanorod composites comprised of carbon and manganese oxide. These new materials have potential applications in fuel cells and metal-air batteries.
Pivotal roles
The oxygen reduction reaction (ORR) is a fundamental reaction that is of great importance in various fields, such as the biological respiration, energy conversion and materials corrosion. Oxygen evolution (OER) is its reverse reaction which is equally significant in photosynthesis and water splitting.
Today, both reactions play pivotal roles in the emerging energy conversion and storage devices including reversible fuel cells and metal-air batteries. However, an efficient ORR/OER process requires costly and rare noble-metal catalysts. Adding to this, good ORR catalysts often lack OER activity and vice versa. This is problematic when switching the charging/discharging mode of the batteries or transforming fuel cells into electrolysis cells. Thus, inexpensive bifunctional ORR/OER catalysts feature high on industrial wish lists.
Outperforming noble metals
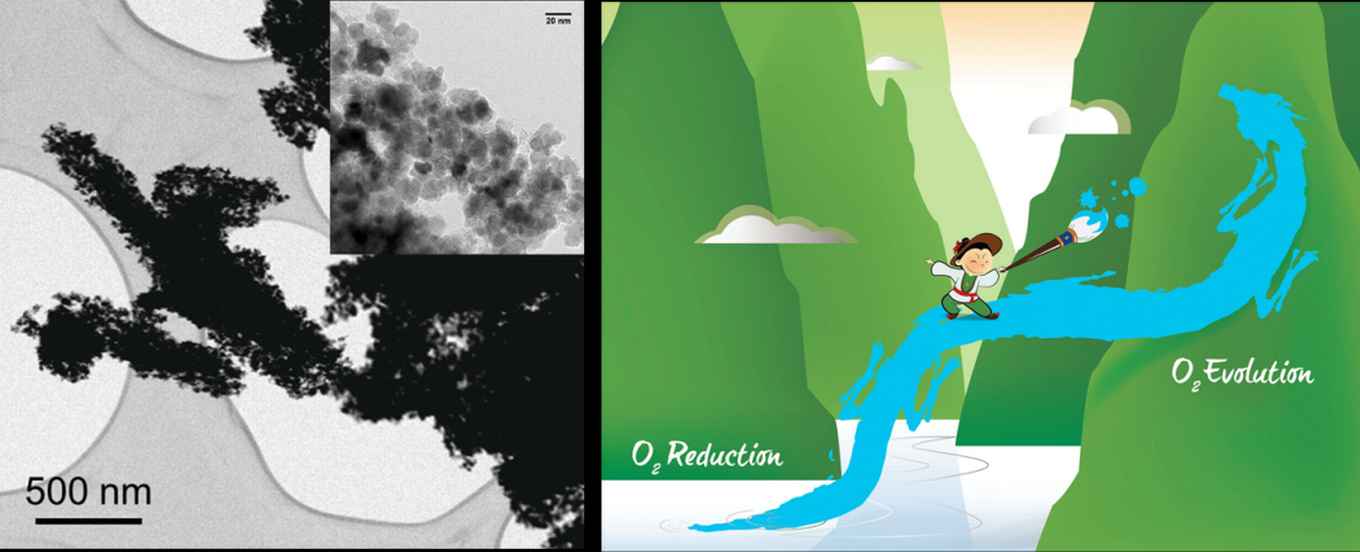
Using two simple and abundant precursors (nitrilotriacetic acid and manganese acetate), Yan and co-workers created a composite nanorod comprising manganese oxides and nitrogen-doped carbon. The carbon matrix forms a contiguous 3D network, connecting all the isolated MnOx nanoparticles and ensuring superior electrical conductivity. The fine and well dispersed manganese nanoparticles provide plenty of bifunctionally active sites. The readily obtained nanorods are actually less active before “baking” at 200 °C. This gives the surface manganese various oxidation states that place this hybrid among the best non-noble-metal ORR/OER catalysts today, outperforming even Pt and RuO2 catalysts.
Emerging investigators special issue
The results were selected and featured in the inaugural Emerging Investigators themed issue of RSC’s renowned journal Green Chemistry, highlighting excellent research carried out by researchers in the early stages of their independent career. Or, in the phrasing of Green Chemistry: articles 'by the rising stars of the green chemistry field'.
Paper:
J. Pandey, B. Hua, W. Ng, Y. Yang, K. van der Veen, J. Chen, N.J. Geels, J.-L. Luo, G. Rothenberg, and N. Yan: Developing hierarchically porous MnOx/NC hybrid nanorods for oxygen reduction and evolution catalysis. Green Chem., 2017,19, 2793-2797. DOI: 10.1039/C7GC00147A