Jacobus Henricus van 't Hoff

A highly influential theoretical chemist of his time, Van 't Hoff helped to found the modern theory of chemical affinity, chemical equilibrium, chemical kinetics, and chemical thermodynamics. Van 't Hoff formulated the theory of the tetrahedral carbon atom and laid the foundations of stereochemistry. He predicted the correct structures of allenes and cumulenes as well as their axial chirality. He is also widely considered as one of the founders of the discipline that is currently called physical chemistry.
After obtaining his diploma from the Delft Polytechnic School in 1871, Van 't Hoff decided to follow a purely scientific career. Based on his experience during vacation work at a sugar factory, he anticipated a dreary profession as a technologist. He enrolled at Leiden University, where he spent a year mainly devoted to mathematics. He then moved to Bonn to work with A. F. Kekulé (1872-1873) and a year later to Paris to work with A.Wurtz (1873-1874). In 1874, he obtained his doctor's degree with E. Mulder of Utrecht University, The Netherlands.
The foundations of stereochemistry
Even before obtaining his doctorate, Van 't Hoff had already published his groundbreaking ideas on stereochemistry in a booklet entitled Voorstel tot Uitbreiding der Tegenwoordige in de Scheikunde gebruikte Structuurformules in de Ruimte, benevens een daarmee samenhangende Opmerking omtrent het Verband tusschen Optisch Actief Vermogen en chemische Constitutie van Organische Verbindingen. In English this translates as Proposal for the Expansion of Structural Formulas used in Space nowadays in Chemistry, as well as a related Note on the Relationship between Optically Active Power and Chemical Constitution of Organic Compounds.
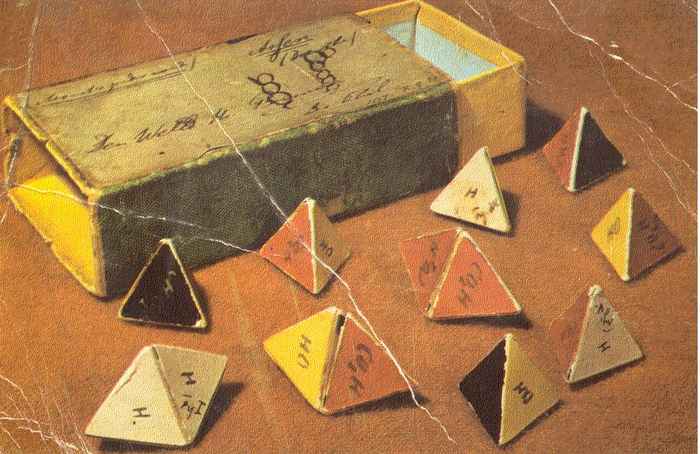
The booklet contained only 13 pages, but would in time have a huge impact on chemical thinking and gave the impetus to the development of stereochemistry. The concept of the “asymmetrical carbon atom” supplied an explanation of the occurrence of numerous isomers, inexplicable by means of the then current structural formulae. Adding to this, Van 't Hoff pointed out the relationship between the optical activity of a compound and the presence of an asymmetrical carbon atom in its molecular structure. The French translation La Chimie dans l'Espace was published in 1875, and in particular after the German translation followed two years later, Van 't Hoffs revolutionary ideas started to take hold. An English translation was published in 1891.
Van 't Hoff in Amsterdam
In spite of his groundbreaking ideas, Van 't Hoff at first struggled to find a job. In 1876 he was finally appointed as lecturer at Utrecht Veterinary College. The following year he moved to the University of Amsterdam (UvA) for a similar position. In 1878 he was appointed full UvA professor at the chair of Chemistry, Mineralogy, and Geology. That same year he married Johanna Francina Mees, with whom he would have two sons and two daughters.

Van 't Hoff's first Amsterdam laboratory was at Groenburgwal 44 in the historic city centre. Here he performed the research for his book Études de Dynamique chimique (Studies in dynamic chemistry), published In 1884. It contains his first explorations of the field of physical chemistry. Of great importance was Van 't Hoff's development of the general thermodynamic relationship between the heat of conversion and the displacement of the equilibrium as a result of temperature variation. In 1885, this principle of mobile equilibrium was put in a more general form by Le Chatelier, hence its current status as the Van ‘t Hoff-Le Chatelier principle.
In 1885, Van 't Hoff published L’Équilibre chimique dans les Systèmes gazeux ou dissous à I’État dilué, which dealt with the theory of dilute solutions - the title translates as Chemical equilibria in gaseous systems or strongly diluted solutions. Here he demonstrated that, in solutions which are sufficiently dilute, the “osmotic pressure” is proportionate to the concentration and the absolute temperature. He postulated that this pressure can be represented by a formula which only deviates from the formula for gas pressure by a coefficient i, and he determined the value of i by various methods. Thus van ‘t Hoff was able to prove that thermodynamic laws are not only valid for gases, but also for dilute solutions. His pressure laws, given general validity by the electrolytic dissociation theory of Svante Arrhenius (1884-1887) – the first foreigner who came to work with him in Amsterdam (1888) – are considered the most comprehensive and important in the realm of natural sciences.

Van 't Hoff's reputation attracted many students from near and far. Frustrated with the duties of lectures, exams and supervision, in 1896 Van 't Hoff gave in to an offer from the Prussian Academy of Sciences that enabled him to fully devote himself to research. He moved to Berlin, where he worked until his death on 1 March 1911.
In 1901, Van 't Hoff was awarded the very first Nobel Prize in Chemistry "in recognition of the extraordinary services he has rendered by the discovery of the laws of chemical dynamics and osmotic pressure in solutions".
Sources:
- Nobel Lectures, Chemistry 1901-1921, Elsevier Publishing Company, Amsterdam, 1966. Online available at Nobelprize.org.
- Wie was Jacobus Henricus van 't Hoff? Biography published at the website of the Royal Netherlands Academy of Arts and Sciences.
- Van’t Hoff’s Molecular Paper Models at dataphys.org.
- Rijksmuseum Boerhaave
- Wikipedia page regarding Jacobus Henricus van 't Hoff.
More information on Van 't Hoff, predominantly in Dutch, can be found at the website of the Chemie Historische Groep (History of Chemistry group) of the Royal Netherlands Chemical Society KNCV.