A new mode of chemical reactivity for metal–free hydrogen activation by Lewis acidic boranes
16 April 2019
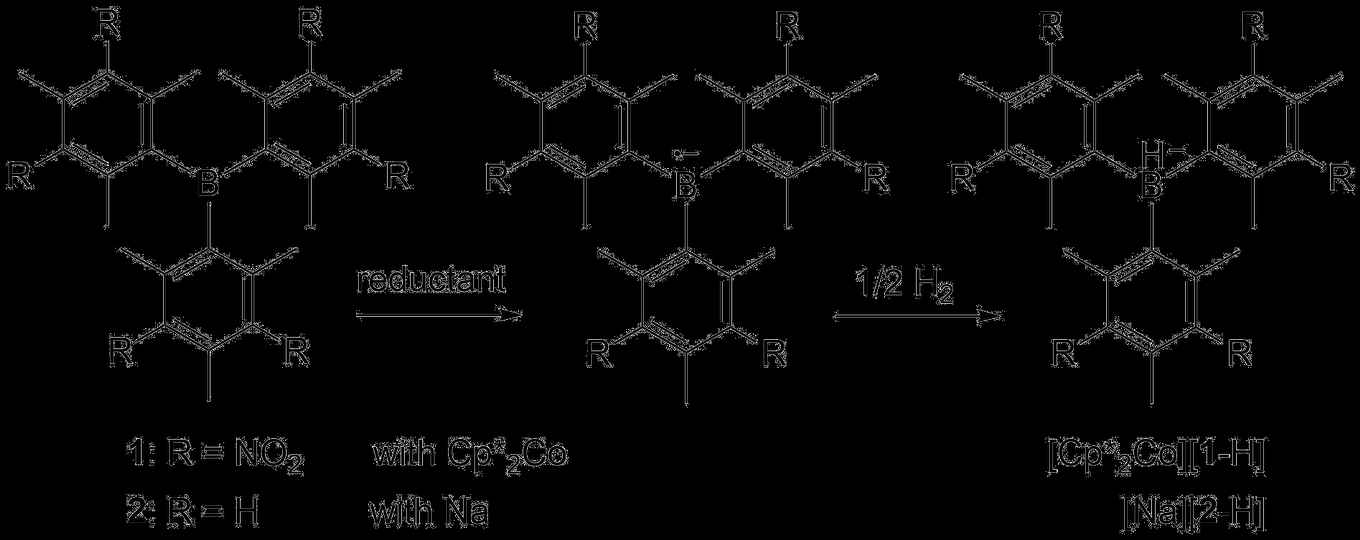
In their paper, Ehlers, Slootweg and colleagues reveal a completely new mode of reactivity for boranes in dihydrogen splitting. Since the seminal discovery of Doug Stephan in 2006 of the transition-metal-free activation of dihydrogen, a variety of catalytic hydrogenation reactions were developed by using pairs of Lewis bases (like phosphines) and Lewis acids (like boranes). The new findings show that by one-electron reduction stable borane radical anions can be obtained that are also capable of splitting dihydrogen homolytically, without the use of any exogenous Lewis bases.
Publication details:
Elliot L. Bennett, Elliot J. Lawrence, Robin J. Blagg, Anna S. Mullen, Fraser MacMillan, Andreas W. Ehlers, Daniel J. Scott, Joshua S. Sapsford, Andrew E. Ashley, Gregory G. Wildgoose, and J. Chris Slootweg: A New Mode of Chemical Reactivity for Metal–Free Hydrogen Activation by Lewis Acidic Boranes. Angewandte Chemie Int. Ed., First published: 09 April 2019. DOI: 10.1002/anie.201900861