Amsterdam researchers develop novel single-atom catalyst for efficient water oxidation
Novel concept holds promise of extremely efficient use of noble metals in catalysis
18 October 2019
To combat the problem of CO2 emissions and decrease the dependency on fossil fuels, the world is turning towards renewable energy that is usually generated as electricity using sunlight or wind. Such sources, however, display intermittency that is not in sync with the fluctuating demand. Therefore, storage solutions have to be developed that allow the use of renewable energy at any desired point in time.
A promising storage option is using renewable electricity to produce hydrogen gas or synthetic fuels. This involves electrocatalytic water splitting that converts electrical energy into oxygen (O2) and hydrogen (H2). The latter can serve as a fuel directly, or as a feedstock for the chemical industry to produce synthetic fuels or other chemicals (e.g. for the production of plastics and other products).
Improving catalysts for oxygen generation
To minimize conversion losses, it is crucial to optimise the water-splitting process and to enhance the efficiencies of the hydrogen and oxygen evolution reactions (HER and OER).
In particular, the oxygen evolution process is relatively complex. It involves four proton-coupled-electron transfer steps proceeding via several different intermediates. Because of this, catalyst optimization aimed at enhancing conversion rates is quite complicated. Current OER catalysts in many water splitting systems, therefore, operate at relatively high overpotentials, which lowers the efficiency of the overall process.
Now, following a single-atom approach, chemists at the University of Amsterdam have developed a novel OER catalyst with excellent performance. The catalyst was developed in a joint effort within the university's Research Priority Area Sustainable Chemistry, involving research groups in homogeneous and heterogeneous catalysis, and computational chemistry.
Beyond the level of today's OER catalysts
The discovery of the new material was based on a collaborative project led by Dr Ning Yan and Prof. Joost Reek, which originally aimed at developing an immobilized molecular electrocatalyst. The presence of molecular catalytic species, however, did not improve the catalytic performance. In contrast, after a pyrolysis step the current single-atom material was obtained, which did improve catalysis - even beyond the level of today’s typical OER catalysts. Together with Prof. Evert Jan Meijer and Nitish Govindarajan, MSc, both from the computational chemistry group, a deeper understanding of the catalysis mechanism was acquired. The results were recently published in the Journal of Materials Chemistry A.
According to the Amsterdam researchers, their novel catalyst concept paves the way to future commercial application of single-atom catalysts in water splitting. In the meantime, it provides a model system for designing catalysts at the atomic scale, enabling extremely efficient use of rare noble metals and boosting their catalytic performance. Not just for water splitting, but also for other catalytic reactions.
Hybrid catalyst materials
A good OER catalyst must combine many features. It has to display excellent intrinsic activity at low overpotential, display adequate electrical conductivity, possess plentiful active sites, and be very stable as well as cost-effective. Since it is nearly impossible to achieve all this in a single material, hybrid catalyst systems are explored. Typically, earth-abundant transition metal oxides are combined with carbon-based support materials. The latter ensures a smooth electron transfer, while the metal oxides display a good OER activity and are cost-effective.
Previously, the Amsterdam researchers have designed a variety of OER catalysts applying a supramolecular approach based on the use of transition metal oxides and molecular iridium carbene complexes. These catalysts show high reaction rates at low overpotential. For the complex implementation in devices, they also demonstrated their immobilization in carbon nanotubes via a functionalisation with pyrene moieties.
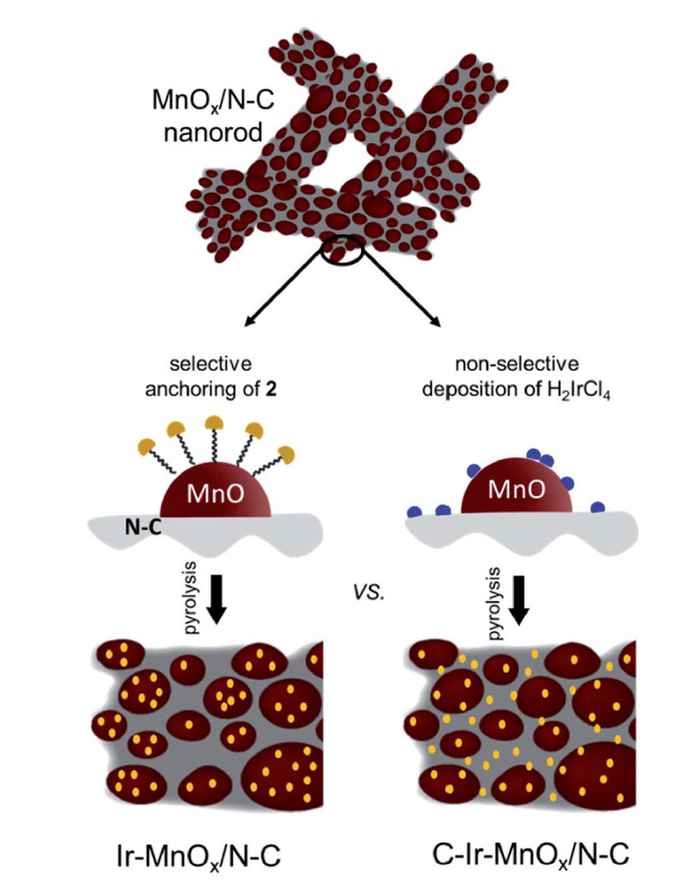
Now, in their Journal of Materials Chemistry A paper, they describe how they have implemented the findings of recent catalysis research showing that the incorporation of site-isolated, single-atom noble metals can boost catalytic activity while minimizing cost. In particular, the supramolecular approach of the Amsterdam researchers offers a solution to the problem that single atoms are intrinsically prone to agglomeration and/or ripening.
They were able to selectively anchor mononuclear Ir-complexes to the metal oxide of a typical OER composite catalyst comprising nitrogen-doped carbon (N–C) and MnOx. To this end, a typical trimethoxysilane functional group was installed onto the molecular iridium carbene complex. Grafting this complex followed by pyrolysis enables highly selective and uniform deposition of Ir atoms on MnOx, providing a superior water oxidation catalyst material.
The obtained composite material shows water oxidation catalysis at 10 mAcm-2, with an overpotential as low as 250 mV, and excellent stability. DFT calculations show that the origin of the enhanced catalyst performance is in the cooperative action of the isolated Ir atom and the surrounding MnO material. The researchers conclude that their approach offers great potential for the design of a range of hybrid materials to be used in water oxidation and, beyond that, in a whole range of energy and catalysis applications.
Publication details
Ning Yan, Remko J. Detz, Nitish Govindarajan, Jacobus M. Koelewijn, Bin Hua, Peng Li, Evert Jan Meijer and Joost N. H. Reek: Selective surface functionalization generating site-isolated Ir on a MnOx/N-doped carbon composite for robust electrocatalytic water oxidation. J. Mater. Chem. A, 2019,7, 23098-23104. DOI: 10.1039/C9TA08447A