Titanium-catalyzed esterification reactions: beyond Lewis acidity
Paper in ChemCatChem
27 July 2020
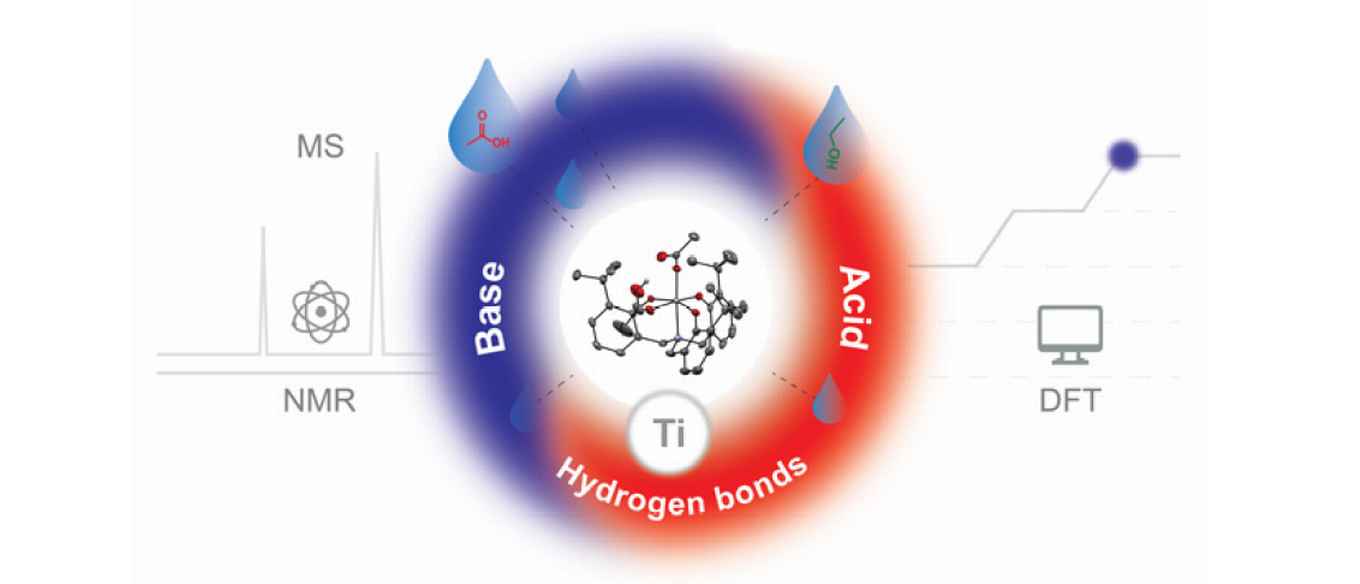
Abstract:
Esterification is a key reaction and used in many synthetic and industrial processes, yet the detailed mechanism of operation of often‐used (Lewis acid) catalysts is unknown and subject of little research. Here, we report on mechanistic studies of a titanium aminotriphenolate catalyst, using stoichiometric and catalytic reactions combined with kinetic data and density functional theory (DFT) calculations. While often only the Lewis acidity of the Ti‐center is taken into account, we found that the amphoteric nature of this catalyst, combining this Lewis acidity with Brønsted basicity of a Ti‐bound and in situ formed carboxylate group, is crucial for catalytic activity. Furthermore, hydrogen bonding interactions are essential to pre‐organize substrates and to stabilize various intermediates and transition states and thus enhancing the overall catalytic reaction. These findings are not only applicable to this class of catalysts, but could be important for many other esterification catalysts.
Paper:
Lukas A. Wolzak, Jarl Ivar van der Vlugt, Keimpe J. van den Berg, Joost N.H. Reek, Moniek Tromp, Ties J. Korstanje: Titanium‐catalyzed esterification reactions: beyond Lewis acidity. ChemCatChem, first published 13 July 2020. DOI: 10.1002/cctc.202000931