A Platinum(II) Metallonitrene with a Triplet Ground State
24 August 2020
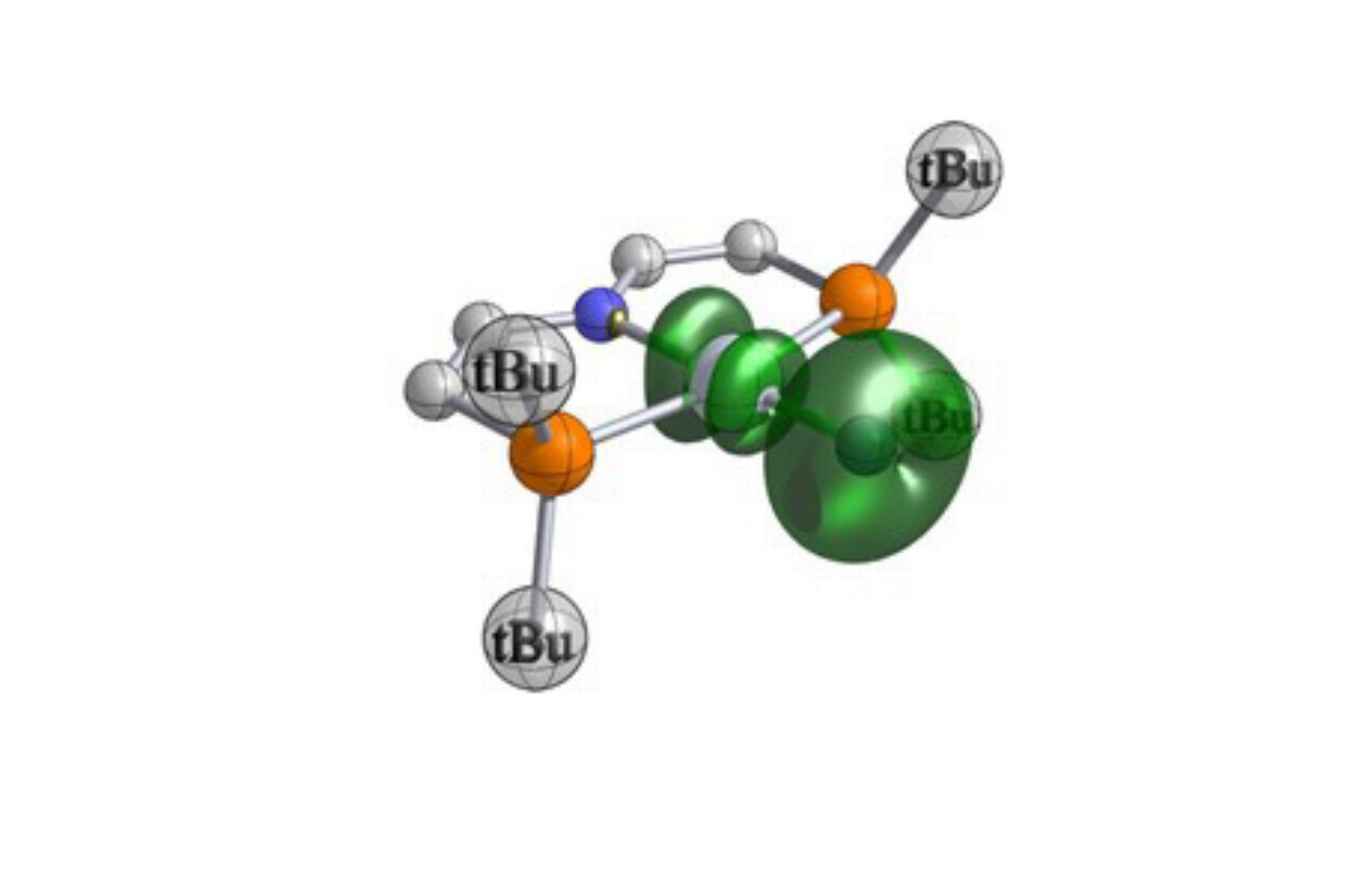
Abstract
Metallonitrenes (M–N) are complexes with a subvalent, atomic nitrogen ligand that have been proposed as key reactive intermediates in nitrogen atom transfer reactions. However, in contrast to the common class of nitride complexes (Mo≡N) and organic nitrenes (R–N), authentic, persistent metallonitrenes remain elusive. We here report that the photolysis of a platinum(II) pincer azide complex enabled the crystallographic, spectroscopic, magnetic and computational characterization of a metallonitrene that is best described as a singly bonded, atomic nitrogen diradical ligand bound to platinum(II). The photoproduct exhibits selective C–H, B–H, and B–C nitrogen atom insertion reactivity. Mechanistic examination of aldehyde C–H amidation surprisingly reveals nucleophilic reactivity of the subvalent N-diradical ligand.
Research cooperation
The contribution of Prof. Bas de Bruin to the research stems from a long-time cooperation with Prof. Sven Schneider of the University of Göttingen, in the field of the reactivity of open-shell organometallic compounds, amido's, nitrido's, nitrenes, and N2 activation.
Paper
Sven Schneider, Jian Sun, Josh Abbenseth, Hendrik Verplancke, Martin Diefenbach, Bas de Bruin, David Hunger, Christian Würtele, Joris van Slageren, Max C. Holthausen: A Platinum(II) Metallonitrene with a Triplet Ground State. Nature Chemistry, 24 August 2020, DOI: 10.1038/s41557-020-0522-4