UvA Chemists Design New Catalysts for Converting CO2 to Useful Chemicals
2 November 2020
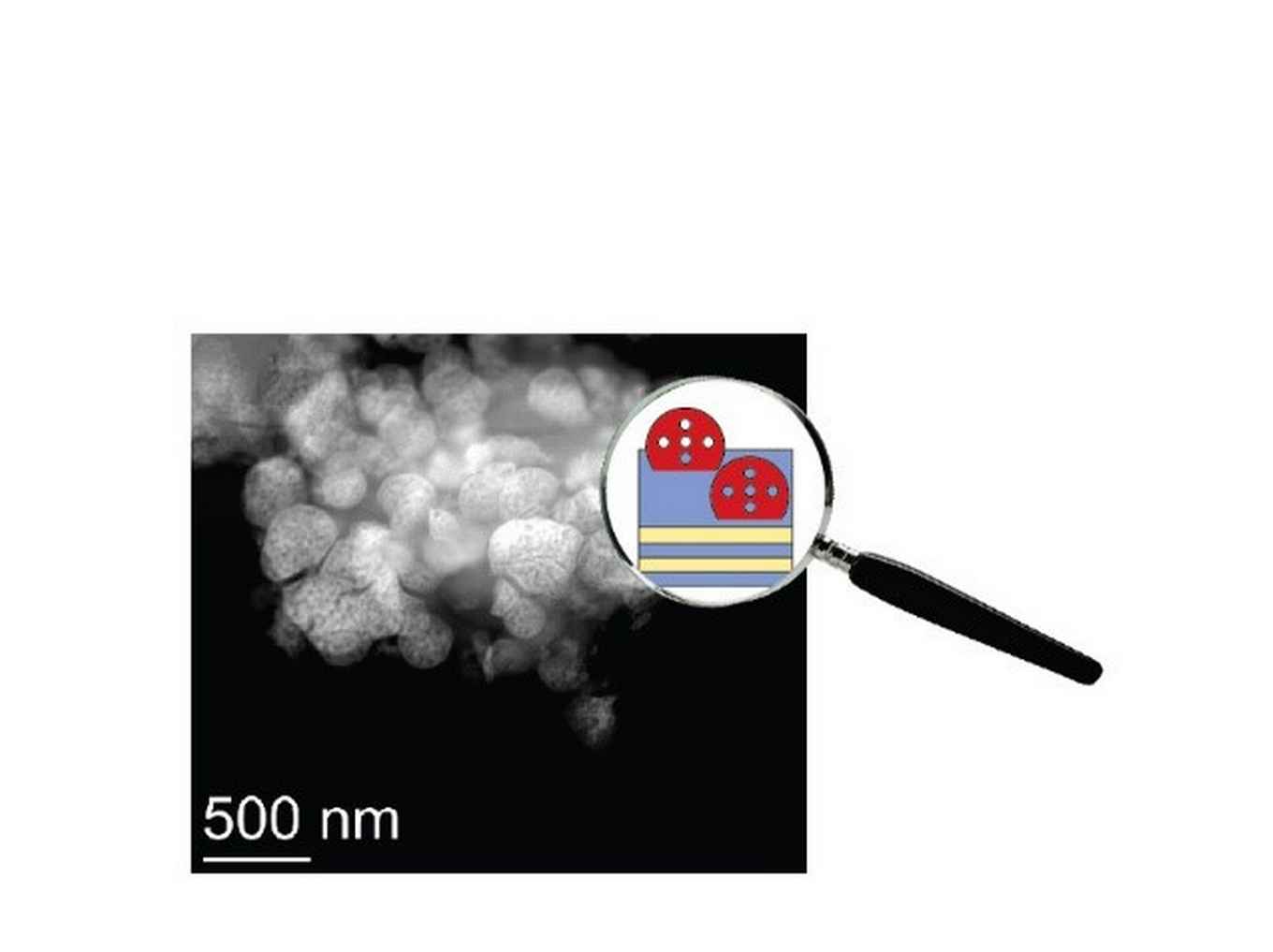
Carbon dioxide emissions are implicated as the chief culprit in climate change scenarios. Yet throwing away this carbon is wasteful and unnecessary. Instead, CO2 should be thought of as a valuable C1 feedstock for making chemicals and fuels. With this approach in mind, PhD student Maria Ronda Lloret has designed and studied new and efficient catalysts for CO2 valorisation, as she now reports in a special issue on “Green Carbon Science: CO2 Capture and Conversion” of the international journal ChemSusChem.
Ronda Lloret, together with supervisors Dr Raveendran Shiju (CatEng) and Prof. Gadi Rothenberg (HCSC) studied the thermocatalytic conversion of CO2 via dry reforming of butane, a light alkane. This reaction produces syngas (a mixture CO and hydrogen) which can be used as a feedstock for making hydrocarbons or alcohols through the Fischer-Tropsch synthesis. In this way, the carbon from the CO2 molecules is re-cycled into useful chemical products.

The researchers focused on MAX phases. These are layered ternary carbides or nitrides that feature an unusual set of properties. Like ceramics, they show high thermal stability, but they are also tough, ductile, and conduct electricity and heat like metals. Recognising the potential of MAX phases in catalysis, in 2018, Shiju and Rothenberg applied them in oxidative dehydrogenation catalysis. Now, Ronda Lloret, studied the potential of MAX phase supported cobalt catalysts for CO2 reforming of butane to syngas. She showed that the properties of a Ti2AlC MAX phase play an important role in catalysis when used as catalyst support. The catalyst was active and selective to CO and hydrogen from 450 °C and above. In addition, the material showed excellent stability over time. Benchmark materials, such as cobalt oxide supported on Al2O3, TiO2 and TiC, strongly deactivated under the same conditions due to carbon poisoning, particle sintering or thermal decomposition. This shows also the potential of MAX phase to act as a support at high temperature reactions (> 400 °C).
This research was supported by a NWO-NSFC joint grant, and was carried out in collaboration with TU/Delft (The Netherlands), UCLouvain (Belgium), Cadiz University (Spain) and Alicante University (Spain). The results are published as an invited open-access paper in ChemSusChem, as part of a Special Issue entitled “Green Carbon Science: CO2 Capture and Conversion”. The authors hope that this study encourages more researchers to apply these fascinating materials in catalysis.
Open-access article
M. Ronda‐Lloret, V. S. Marakatti, W. G. Sloof, J. J. Delgado, A. Sepúlveda‐Escribano, E. V. Ramos‐Fernandez, G. Rothenberg, N. R. Shiju: Butane Dry Reforming Catalyzed by Cobalt Oxide Supported on Ti2AlC MAX Phase, ChemSusChem 2020, 13, EarlyView.
https://chemistry-europe.onlinelibrary.wiley.com/doi/epdf/10.1002/cssc.202001633