Chemical attribution of fentanyl: The effect of human metabolism
29 March 2021
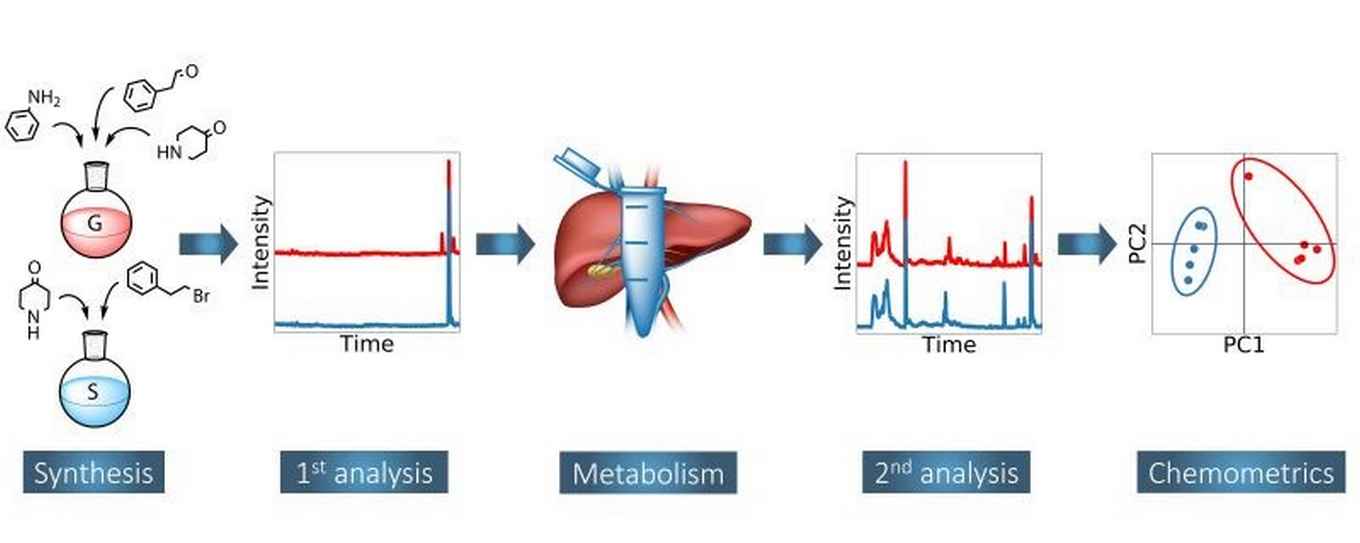
Highlights
- Marker compounds of fentanyl synthesis routes were identified post-metabolism.
- Impurities were detected up to levels relevant in forensic casework.
- Both GC-FID and LC-Orbitrap-MS yield discriminating impurities.
Abstract
Chemical attribution typically aims to establish a link between material found at a crime scene and a person, location or other evidence. In the field of illicit drugs, chemical attribution signatures are usually impurity profiles. Extending these to metabolized samples would create new possibilities in forensic investigations. The present study explores the effect of human metabolism on the impurity profile of fentanyl, as representative of synthetic opioids. Two different methods (Gupta and Siegfried) were used to synthesize fentanyl, after which the samples were incubated with liver microsomes to mimic human metabolism. The impurity profiles have been characterized with gas chromatography-mass spectrometry (GC-MS), gas chromatography with flame ionization detector (GC-FID), liquid chromatography quadrupole-time of flight mass spectrometry (LC-Q-TOF-MS) and liquid chromatography orbitrap mass spectrometry (LC-Orbitrap-MS). It was found that GC-FID and LC-Orbitrap-MS can both be used to discriminate between the Gupta and Siegfried synthesis method. This holds both for the analyses performed before and after metabolism. In addition, principal component analysis (PCA) identified acetyl fentanyl as the most important marker compound. Associated detection limits are in the range of concentrations expected in case work. While acetyl fentanyl is not stable during metabolism, its discriminating potential is transferred to its metabolic product acetyl norfentanyl. In addition, the stable impurities phenylacetamide and 1-phenylethylpiperidin-4-ol were found to be significant classifiers. To implement the results in a forensic framework, linear discriminant analysis (LDA) was applied and used to establish likelihood ratios. To our knowledge, the present work demonstrates for the first time the possibility of chemical attribution of drugs through the analysis of metabolic trace levels in biological samples.
Paper
Mirjam de Bruin-Hoegée, Djarah Kleiweg, Daan Noort, Arian C. van Asten: Chemical attribution of fentanyl: The effect of human metabolism. Forensic Chemistry, Vol 24, June 2021 DOI: 10.1016/j.forc.2021.100330
Project
The research is part of the FACING project, a collaboration between the Van 't Hoff Institute for Molecular Sciences (HIMS) and TNO Defence, Safety & Security financed by the DO-AIO fund of the Dutch Ministry of Defence.