A novel approach to the synthesis of mechanically interlocked peptidic products
17 August 2021
Abstract
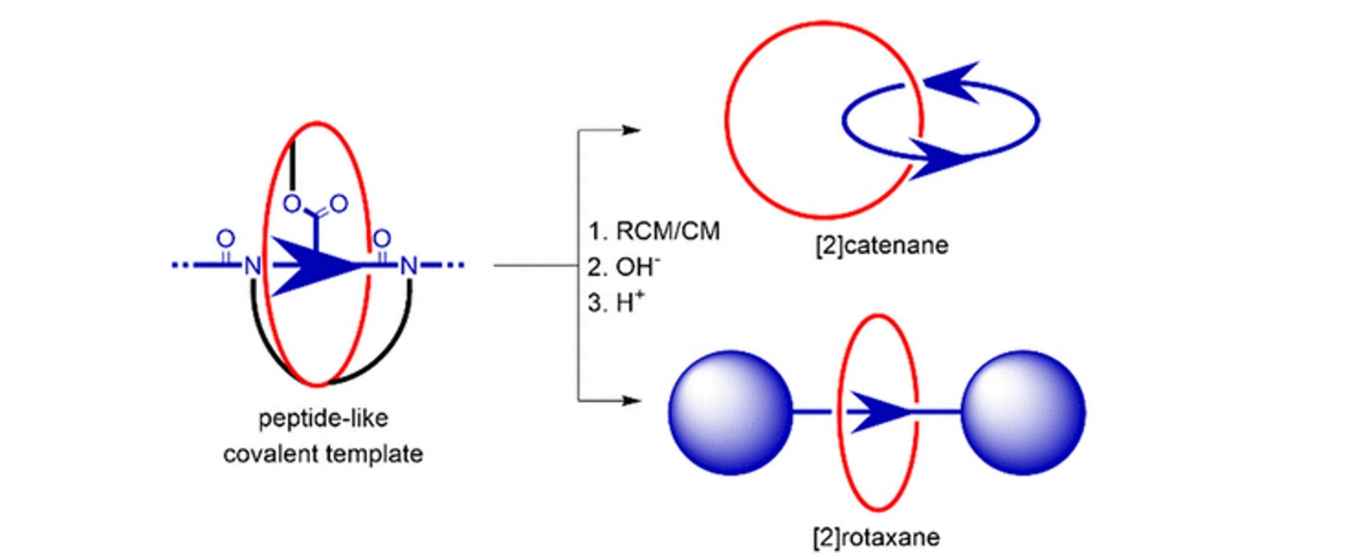
Despite the advances in the synthesis of mechanically interlocked molecules, a generally applicable approach to interlocked natural products, such as lasso peptides, is yet to be formulated. While amino acid sequences have been introduced into several rotaxanes, the key structural components have always been dictated by the method used for supramolecular preorganization. In this work, we report the use of an ester-functionalized, aromatic δ-amino acid as the central covalent templating unit in the synthesis of both a [2]catenane and a [2]rotaxane from the same multimacrocyclic intermediate. This represents a key step toward future synthetic peptide-based interlocked products.
Paper
Simone Pilon, Steen Ingemann Jørgensen, and Jan H. van Maarseveen: Covalent [2]Catenane and [2]Rotaxane Synthesis via a δ-Amino Acid Template. ACS Org. Inorg. Au Publication Date: August 11, 2021, DOI: 10.1021/acsorginorgau.1c00017