Rapid and direct photocatalytic acylation and arylation of alkylic C-H bonds in a flow system
16 August 2021
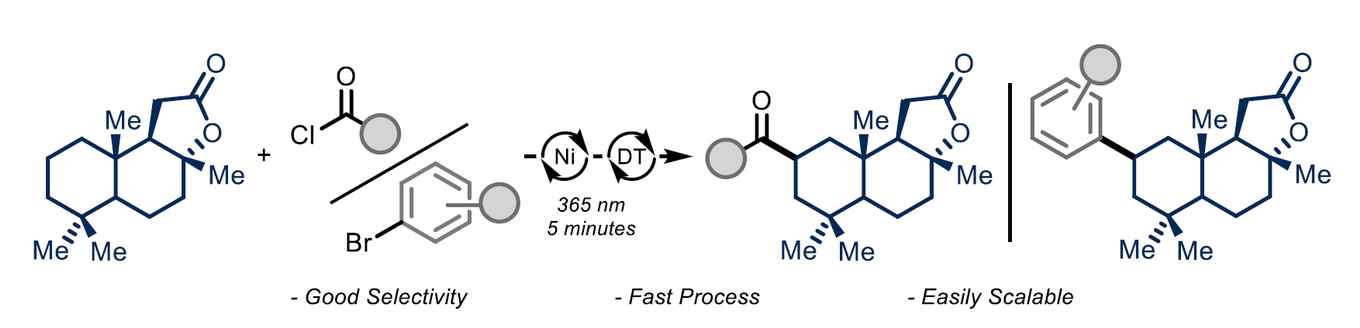
Due to the mild reaction conditions, the methodology shows great functional group tolerance and high regioselectivity, and can be used for the late-stage functionalization of various complex biologically relevant molecules. Kinetic studies show that the combination of a microfluidic environment and powerful light sources boosts the overall rate of the reaction without affecting the mechanism. The study was performed in cooperation with Signify (previously Philips Lighting), the world-leading company in light source development.
Abstract
Herein, we report a photocatalytic procedure that enables the acylation/arylation of unfunctionalized alkyl derivatives in flow. The method exploits the ability of the decatungstate anion to act as a hydrogen atom abstractor and produce nucleophilic carbon-centered radicals that are intercepted by a nickel catalyst to ultimately forge C(sp3)‒C(sp2) bonds. Owing to the intensified conditions in flow, the reaction time can be reduced from 12-48 hours to only 5-15 minutes. Finally, kinetic measurements highlight how the intensified conditions do not change the reaction mechanism but reliably speed up the overall process.
Paper
Daniele Mazzarella, Antonio Pulcinella, Loïc Bovy, Rémy Broersma, and Timothy Noël: Rapid and Direct Photocatalytic C(sp3)‒H Acylation and Arylation in Flow Angewandte Chemie, First published: 30 July 2021, DOI: 10.1002/anie.202108987
Also read
Chemische reacties in flow tot tweehonderd keer sneller (Dutch C2W chemistry news)