Biocatalytic reductive amination in a 3D-printed continuous flow microreactor
4 October 2022
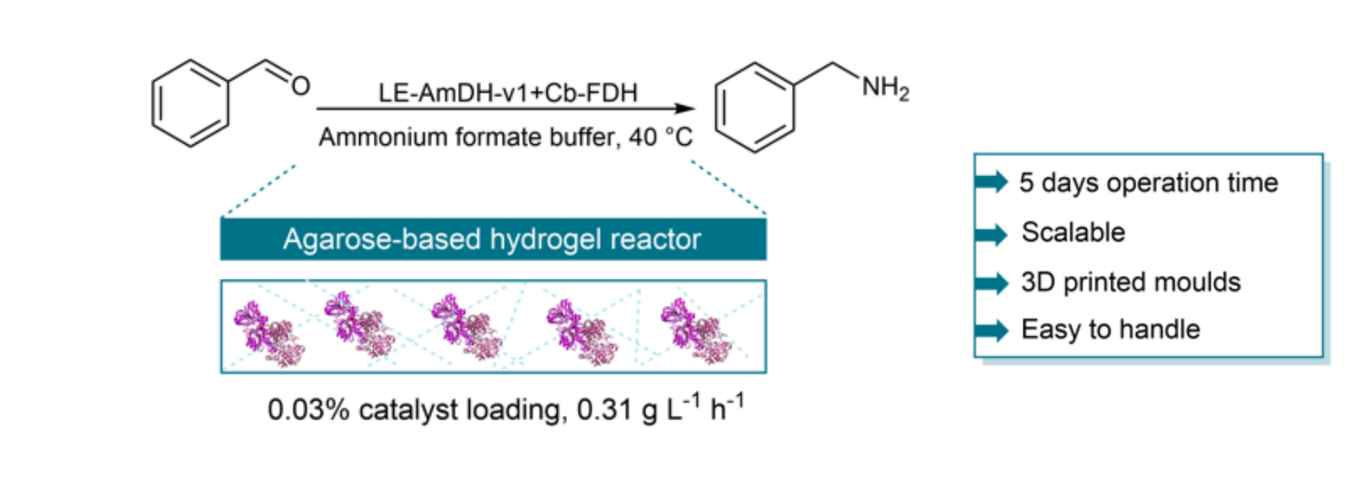
The research led by Dr Francesco Mutti of the Biocatalysis group identified entrapment as the most compatible enzyme immobilisation technique with regard to the reaction conditions for the reductive amination of aldehydes such as benzaldehyde. The joint team then developed a scalable and customisable approach whereby an agarose hydrogel containing the entrapped dehydrogenases is cast in a 3D-printed mould. The work exemplifies how the incorporation of 3D-printing technology in flow biocatalysis can generate simple and efficient solutions that can be rapidly assimilated in chemical manufacturing.
Paper abstract:
Herein, we show how the merge of biocatalysis with flow chemistry aided by 3D-printing technologies can facilitate organic synthesis. This concept was exemplified for the reductive amination of benzaldehyde catalysed by co-immobilised amine dehydrogenase and formate dehydrogenase in a continuous flow micro-reactor. For this purpose, we investigated enzyme co-immobilisation by covalent binding, or ion-affinity binding, or entrapment. Entrapment in an agarose hydrogel turned out to be the most promising solution for this biocatalytic reaction. Therefore, we developed a scalable and customisable approach whereby an agarose hydrogel containing the co-entrapped dehydrogenases was cast in a 3D-printed mould. The reactor was applied to the reductive amination of benzaldehyde in continuous flow over 120 h and afforded 47% analytical yield and a space-time yield of 7.4 g L h-1 using 0.03 mol% biocatalysts loading. This work also exemplifies how rapid prototyping of enzymatic reactions in flow can be achieved through 3D-printing technology.
Paper details:
Federico Croci, Jan Vilím, Theodora Adamopoulou, Vasilis Tseliou, Peter J. Schoenmakers, Tanja Knaus, and Francesco G. Mutti: Continuous flow biocatalytic reductive amination by coentrapping dehydrogenases with agarose gel in a 3D-printed mould reactor. ChemBioChem 2002, e202200549 (first published 29 September 2022). DOI: 10.1002/cbic.202200549
See also
Research group Biocatalysis
Research group Analytical Chemistry