How a single monolayer can have a big impact on catalysis
21 December 2023
Abstract of the paper
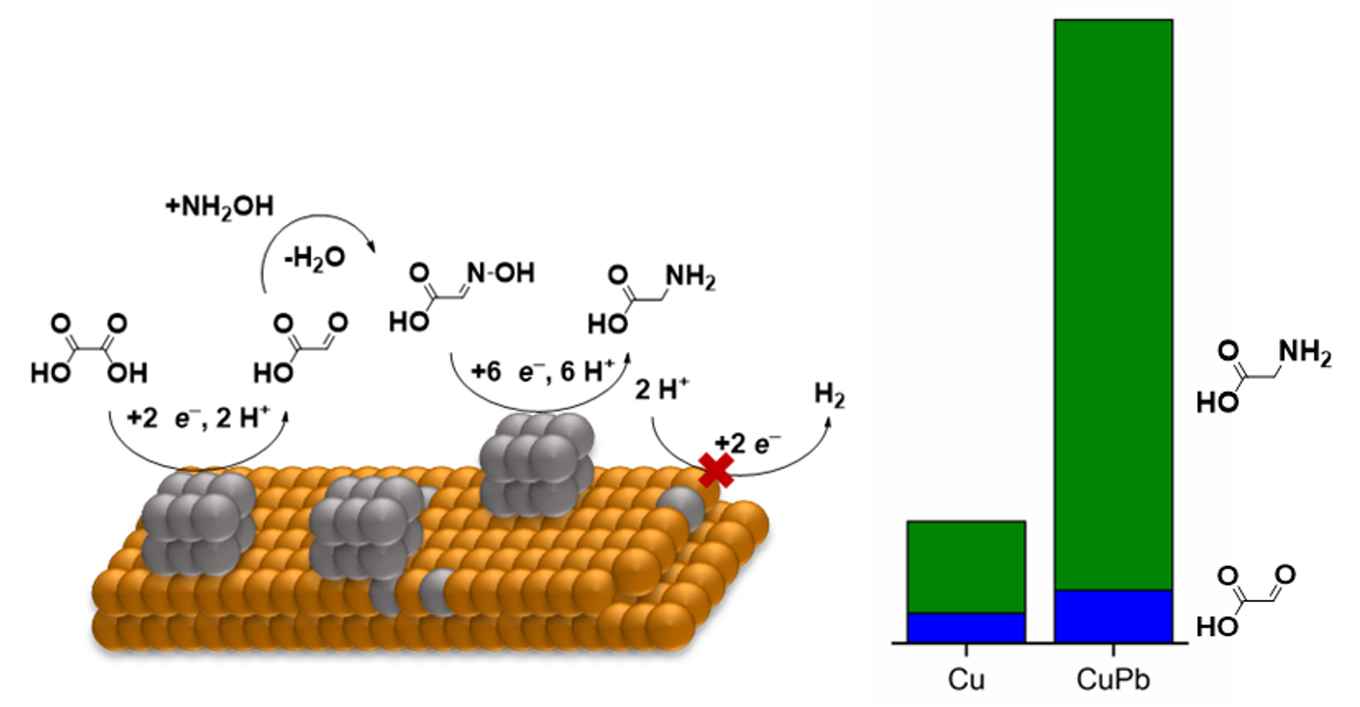
In the field of reductive organic electrosynthesis, the hydrogen evolution reaction (HER) is considered a parasitic reaction that lowers the Faradaic efficiency of the synthesis. Metals with a high overpotential for HER are often used to prevent this. However, this limits the catalytic materials that can be used in these reactions. To expand upon the scope of available electrocatalysts, we prepared a CuPb electrode via underpotential deposition (UPD). We thereby created an electrocatalyst with a single monolayer of Pb, CuPb1ML, in which Pb weight loading is only 415 ng cm-2, yet its properties could still effectively inhibit HER. The CuPb1ML electrode was used in the electrosynthesis of glycine from oxalic acid and hydroxylamine. This reaction served as a model for a C–N bond forming reaction in acidic aqueous media. The CuPb1ML electrode was compared against a pure Pb and Cu metal electrode. The CuPb1ML electrode showed a Faradaic efficiency for glycine production of 57%, which was 9-fold higher than Cu and rivaled the Pb electrode. The catalytic activity of CuPb1ML was 211 µmol h-1 cm-2, which is higher than both Cu and Pb. The mechanism of the electroreduction was then studied via in situ Fourier Transform Infra-Red (FTIR) spectroscopy. These results hinted to an evolution of the electrocatalyst during the electrolysis reaction, which was then studied via Scanning Electron Microscopy (SEM) and X-Ray Photoelectron spectroscopy (XPS). We found that the Pb monolayer restructured during catalysis, forming microparticles that were active in the reaction based on the listed experiments. Pb alloying into the lattice, which can occur during UPD, also lowered the HER, further facilitating glycine synthesis. Thus, our research also shows how Pb UPD impacts the catalytic properties of a metal both through monolayer deposition as well as surface alloying.
Paper details
Pim Broersen, Thijs de Groot, Didjay Bruggeman, Emma Caarls, Jamie Trindell, Dimitra Anastasiadou, Marta Figueiredo, Gadi Rothenberg, Amanda C. Garcia, Enhancing Electrocatalytic Synthesis of Glycine with CuPb1ML Electrode Synthesized via Pb UPD. ChemCatChem 2023, e202301370. DOI: 10.1002/cctc.202301370
See also
Research group Heterogeneous Catalysis and Sustainable Chemistry