Efficient synthesis of biaryl scaffolds through smart catalyst design
21 March 2024
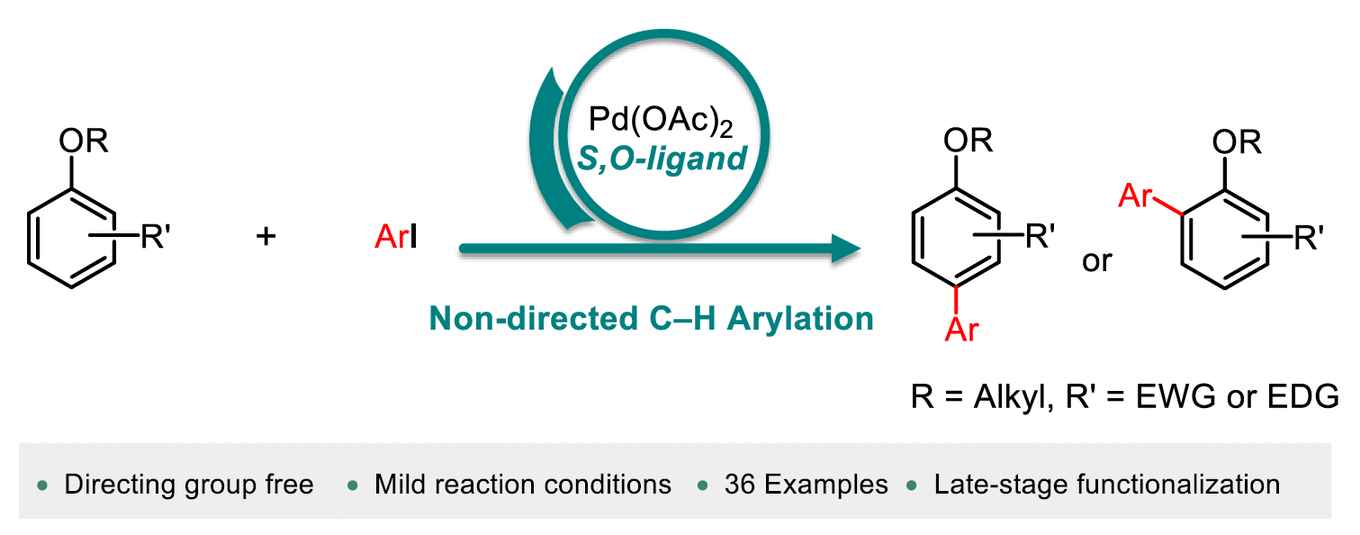
Biaryl scaffolds are of great importance as they are present in a wide range of pharmaceuticals, bioactive molecules, natural products and functional materials. These scaffolds are typically synthesised by coupling two pre-functionalised aryl coupling partners. However, this approach generates a large amount of waste. In recent years, great efforts have been made to improve the sustainability of these transformations by using the C-H bond as a reactive functional group, eliminating the use of pre-functionalised arenes, resulting in a more atom- and step-economic process. Nevertheless, the reported strategies have severe limitations in terms of reaction conditions, availability of arylating agents and scope. The Fernández-Ibáñez group has developed an efficient method for the direct C-H arylation of anisoles using a highly active Pd/S,O ligand catalytic system discovered in their group.
Abstract of the paper
Non-directed C–H arylation is one of the most efficient methods to synthesize biaryl compounds without the need of the prefuctionalization of starting materials, or the installment and removal of directing groups on the substrate. A direct C–H arylation of simple arenes as limiting reactants remains a challenge. Here we disclose a non-directed C–H arylation of anisole derivatives as limiting reagents with aryl iodides under mild reaction conditions. The arylated products are obtained in synthetically useful yields and the arylation of bioactive molecules is also demonstrated. Key to the success of this methodology is the use of a one-step synthesized S,O-ligand.
Paper details
Ke-Zuan Deng, Verena Sukowski, Maria Ángeles Tati Fernández-Ibáñez: Non-Directed C–H Arylation of Anisole Derivatives via Pd/S,OLigand Catalysis. Angewandte Chemie International Edition, e202400689, 24 February 2024. DOI: 10.1002/anie.202400689