Efficient and rapid cross-coupling of aryl bromides with alkyl boranes
16 May 2024
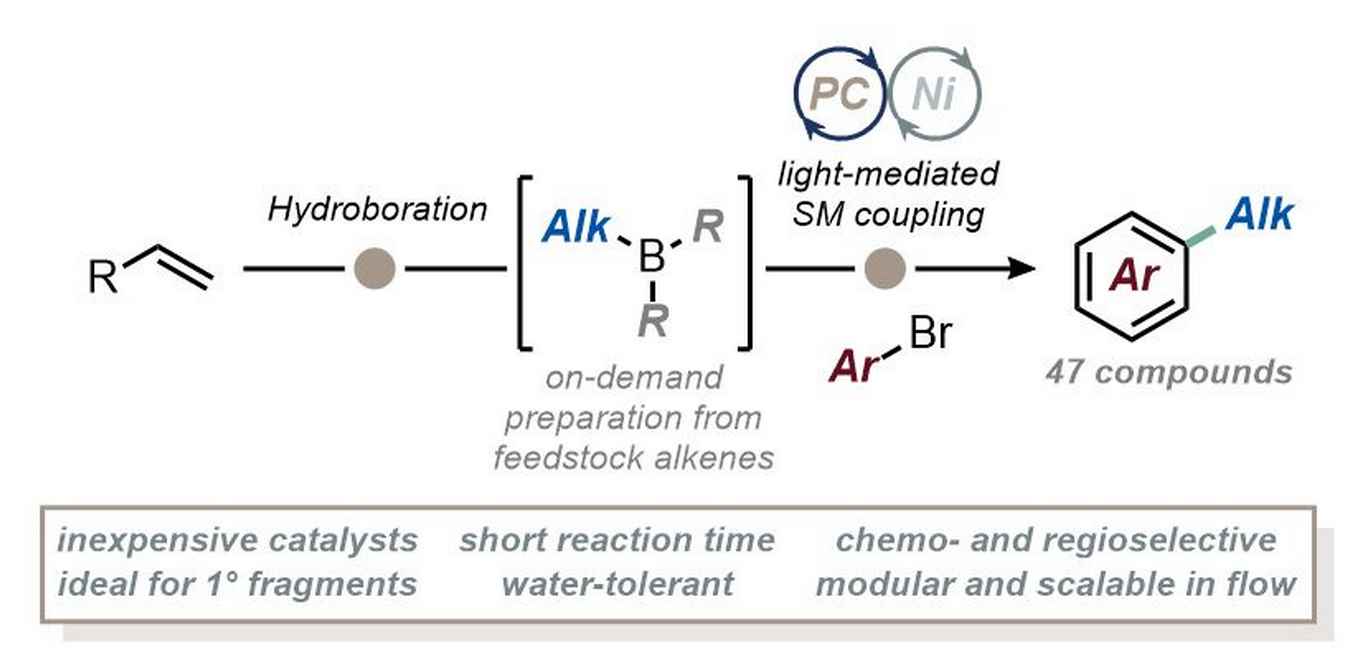
Leveraging the classical hydroboration techniques pioneered by Nobel Laureate H.C. Brown, the new approach simplifies the introduction of aliphatic fragments into aromatic compounds, most notably primary aliphatic fragments. The straightforward synthesis is ideal for functionalizing biologically relevant alkyl substrates in medicinal chemistry and total synthesis. It operates efficiently at room temperature, completing in just 30 minutes without the need for expensive catalysts or sensitive organometallics. Flow technology enabled the integration of hydroboration and cross-coupling in one uninterrupted process, significantly enhancing scalability, safety, and overall efficiency.
Abstract of the paper
In recent years, there has been a growing demand for drug design approaches that incorporate a higher number of sp3-hybridized carbons, necessitating the development of innovative cross-coupling strategies to reliably introduce aliphatic fragments. Here, we present a powerful approach for the light-mediated B-alkyl Suzuki−Miyaura cross-coupling between alkyl boranes and aryl bromides. Alkyl boranes were easily generated via hydroboration from readily available alkenes, exhibiting excellent regioselectivity and enabling the selective transfer of a diverse range of primary alkyl fragments onto the arene ring under photocatalytic conditions. This methodology eliminates the need for expensive catalytic systems and sensitive organometallic compounds, operating efficiently at room temperature within just 30 min. We further demonstrate the translation of the present protocol to continuous-flow conditions, enhancing scalability, safety, and overall efficiency of the method. This versatile approach offers significant potential for accelerating drug discovery efforts by enabling the introduction of complex aliphatic fragments in a straightforward and reliable manner.
Paper details
Ting Wan, Luca Capaldo, Jonas Djossou, Angela Staffa, Felix J. de Zwart, Bas de Bruin & Timothy Noël: Rapid and scalable photocatalytic C(sp2)–C(sp3) Suzuki−Miyaura cross-coupling of aryl bromides with alkyl boranes. Nat Commun 15, 4028 (2024). DOI: 10.1038/s41467-024-48212-5
See also
- Research group Flow Chemistry
- Research group Homogeneous, Supramolecular and Bio-inspired Catalysis