A viable bio-electrocatalytic system for alkene reduction
Using ene-reductases with methyl viologen as electron mediator
26 September 2024
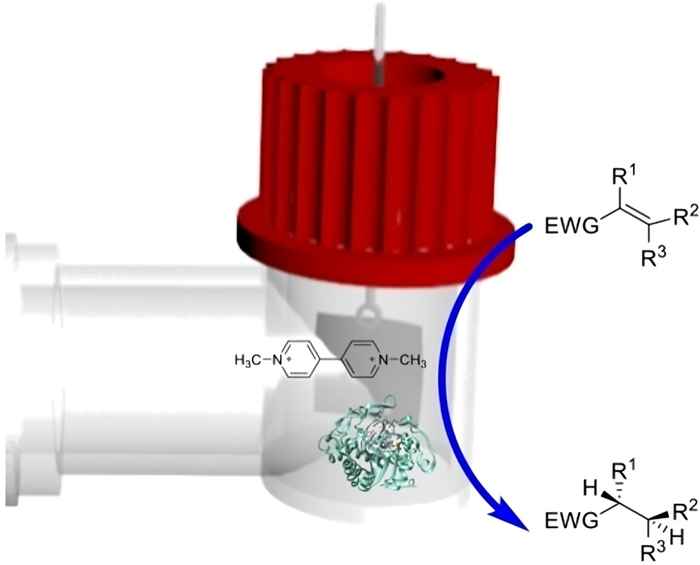
By combining electrochemistry and biocatalysis, the researchers led by associate professor Dr Francesco Mutti solved an important issue that until now has hindered the broad application of ene-reductases for the asymmetric reduction of activated alkenes. They were able to achieve a cost-effective regeneration of flavin mononucleotide cofactor (FMN) that is essential for the working of the flavin-dependent ene-reductase (ERed) enzymes.
In ChemBioChem, they describe how an added electrochemical feature can recycle the flavin cofactor of EReds using methyl viologen as a mediator. The bio-electrocatalytic setup efficiently catalyzes alkene reduction under constant electrochemical potential. The researchers tested two EReds on various substrates and were able to report high productivity, while retaining the enzymes’ enantioselectivity. The study underscores the promise of bio-electrocatalytic asymmetric hydrogenation and shows the potential for extension to other redox enzymatic reactions.
The research forms part of the PhD research of Zheng Wei, who collaborated with colleagues at the Biocatalysis group (Tanja Knaus, Matteo Damian, Yuxin Liu, Francesco Mutti) as well as the research group Heterogeneous Catalysis and Sustainable Chemistry (Cássia Sidney Santana, Gadi Rothenberg, Ning Yan).
Abstract, as published with the paper
Asymmetric hydrogenation of alkene moieties is important for the synthesis of chiral molecules, but achieving high stereoselectivity remains a challenge. Biocatalysis using ene-reductases (EReds) offers a viable solution. However, the need for NAD(P)H cofactors limits large-scale applications. Here, we explored an electrochemical alternative for recycling flavin-containing EReds using methyl viologen as a mediator. For this, we built a bio-electrocatalytic setup with an H-type glass reactor cell, proton exchange membrane, and carbon cloth electrode. Experimental results confirm the mediator's electrochemical reduction and enzymatic consumption. Optimization showed increased product concentration at longer reaction times with better reproducibility within 4–6 h. We tested two enzymes, Pentaerythritol Tetranitrate Reductase (PETNR) and the Thermostable Old Yellow Enzyme (TOYE), using different alkene substrates. TOYE showed higher productivity for the reduction of 2-cyclohexen-1-one (1.20 mM h−1), 2-methyl-2-cyclohexen-1-one (1.40 mM h−1) and 2-methyl-2-pentanal (0.40 mM h−1), with enantiomeric excesses ranging from 11 % to 99 %. PETNR outperformed TOYE in terms of enantioselectivity for the reduction of 2-methyl-2-pentanal (ee 59 % ± 7 % (S)). Notably, TOYE achieved promising results also in reducing ketoisophorone, a challenging substrate, with similar enantiomeric excess compared to published values using NADH.
Paper details
Zheng Wei, Tanja Knaus, Matteo Damian, Yuxin Liu, Cássia S. Santana, Ning Yan, Gadi Rothenberg, Francesco G. Mutti: Bio-electrocatalytic Alkene Reduction Using Ene-Reductases with Methyl Viologen as Electron Mediator. ChemBioChem, Early View, e202400458. DOI: 10.1002/cbic.202400458
See also
Research group HIMS Biocat