Porphyrins on acid: New avenues for using porphyrins in catalysis and photoreactions
26 September 2024
Porphyrins, known from their role in biological processes such as photosynthesis, display unique optical and electronic properties. These arise from their conjugated macrocyclic structure, which allows for extensive π-electron delocalization. It enables porphyrins to transfer energy (as photosensitizers) or electrons (as photoredox catalysts) under light irradiation. They are extensively studied for applications in fields such as materials science, catalysis, energy conversion, and photodynamic therapy.
In a previous study, the researchers at the Homogeneous, Supramolecular and Bio-Inspired Catalysis group explored the working of free-base porphyrin incooperated into a metal-organic framework (MOF), which are known photocatalysts for CO2 reduction. They established that in the confined space of the MOF pore free-base porphyrins undergo partial protonation solely in the presence of water, without further acid needed.
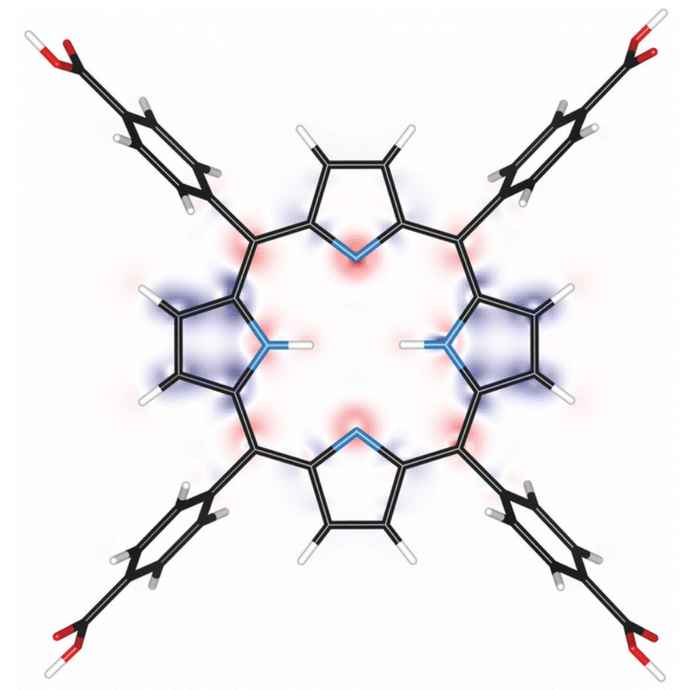
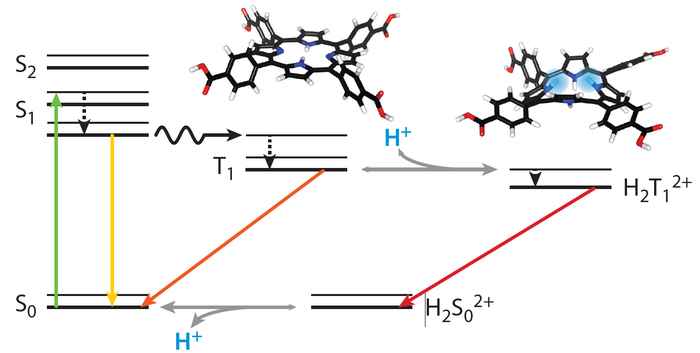
This peculiar finding motivated the researchers to further investigate how partial protonation affects the photoexcited state of tetrakis(4-carboxyphenyl)porphyrin (TCPP). In the PhysChemChemPhys paper thy now report how they used steady-state and nanosecond transient absorption spectroscopy to establish that partially protonated triplet states form from the neutral triplet state, with lifetimes lasting up to 120 μs. This behaviour was confirmed by computational analysis, showing that the excited triplet state of TCPP acts as a photobase, making it more nucleophilic at unprotonated nitrogen atoms. As protonation effects also the redox potentials, this findings open up new avenues for using porphyrins in catalysis and photoreactions.
Abstract, as published with the paper
Free-base porphyrins can be protonated, which significantly impacts their electronic and excited state properties. While excited state dynamics are well explored for either neutral or fully protonated porphyrins, the intermediate region has not yet been explored, although their potential implications for photocatalytic reactions are evident. This study explores how partial protonation affects the nature and properties of photoexcited states of tetrakis(4-carboxyphenyl)porphyrin (TCPP) using steady-state and nanosecond transient absorption spectroscopy. Global-fit analysis of the decay curves revealed the formation of a protonated excited triplet state from the neutral triplet state, as well as the long lifetimes of these species of up to 120 μs. The photoexcited triplet state of TCPP functions as a photobase, which was confirmed by computational analysis of the electron density of the exited states showing increased nucleophilicity at the unprotonated nitrogen atoms of the porphyrin core. These findings indicate that photoinduced protonated excited triplet states can function as electron acceptors with anodically shifted redox potentials, opening new pathways for porphyrin-based photoreactions.
Paper details
P. Tim Prins, Dorota Rutkowska-Zbik, Sonja Pullen and Bettina Baumgartner: Porphyrins on acid: kinetics of the photoinduced-protonation of tetrakis(4-carboxyphenyl)-porphyrin. Phys. Chem. Chem. Phys., 2024, Advance Article. DOI: 10.1039/D4CP02542C