Rigid and tough renewable polyesters to replace ABS and other high performance fossil-based polymers
16 September 2024
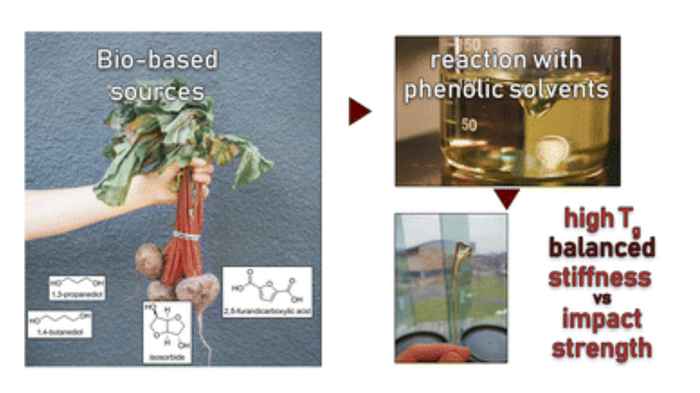
The study was performed in cooperation with the companies Avantium and LEGO and forms part of the research of PhD candidate Bruno Bottega Pergher. It builds on a synthesis method with reactive solvents developed earlier in the group, resulting in aromatic polyesters from (potentially) renewable monomers, using bio-based isosorbide as a means to increase their Tg and to inhibit their crystallization, while using flexible co-diols to improve impact strength. The RSC Sustainability paper reports three promising polyesters resulting from this concept: PIBT (using 1,4-butanediol as co-diol), PICT (with 1,4-cyclohexanedimethanol) and PIPT (with 1,3-propanediol). It shows that in particular the first two show great potential to compete with current high performance polyesters on performance and production cost. They thus enable the substitution of high performance polymers like ABS and polycarbonate with chemically recyclable polyesters from bio-based or recycled sources.
Abstract of the paper
Renewable polyesters with a good balance between impact strength and elastic modulus (stiffness) are not very common, especially when combined with high glass transition temperature (Tg). Achieving such high performance properties would enable the substitution of high performance polymers like ABS and polycarbonate with chemically recyclable polyesters from bio-based or recycled sources. One of the challenges in developing these materials is to select the right composition of the right monomers/comonomer ratios and making these materials with high molecular weight, which can be challenging since some of the most promising rigid diols, such as isosorbide, are unreactive. This study comprises aromatic polyesters from (potentially) renewable monomers, using bio-based isosorbide as a means to increase their Tg and to inhibit their crystallization, while using flexible co-diols to improve impact strength. To incorporate a high amount of isosorbide into the targeted polyesters, the synthesis method with reactive phenolic solvents was used that was previously developed in the Industrial Sustainable Chemistry group. The selected compositions display high Tg's (>90 °C) and high tensile modulus (>1850 MPa). It was shown that more polar monomers such as the stiffer 2,5-furandicarboxylic acid (FDCA) and diethylene glycol cause high stiffness but decreased impact strength (<5 kJ m−2). Combining terephthalic acid and isosorbide with more flexible diols like 1,4-butanediol, 1,4-cyclohexanedimethanol (CHDM) and 1,3-propanediol provides a better balance, including the combination of high tensile modulus (>1850 MPa) and high impact strength (>10 kJ m−2).
Paper details
Bruno Bottega Pergher, Daniel H. Weinland, Robert-Jan van Putten, and Gert-Jan M. Gruter: The search for rigid, tough polyesters with high Tg – renewable aromatic polyesters with high isosorbide content. RSC Sustain., 2024, 2, 2644-2656. DOI: 10.1039/D4SU00294F
See also
Industrial Sustainable Chemistry group