Unravelling the workings of ‘miracle solvent’ hexafluoro-isopropanol
21 October 2024
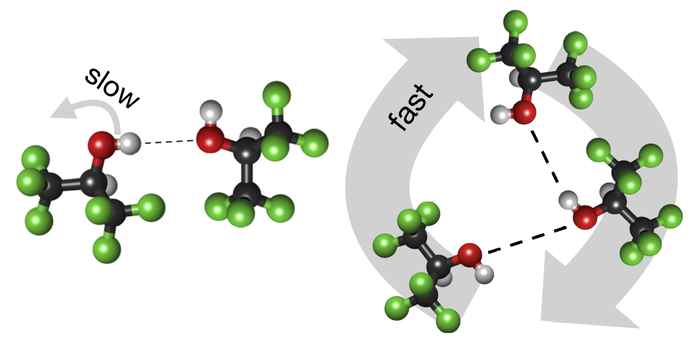
The international research team investigated the hitherto barely explored hydrogen-bond dynamics of HFIP in comparison to its non-fluorinated analogue isopropanol. They established that hydrogen-bond fluctuations and the reorientation of individual OH groups (as probed by time-resolved infrared spectroscopy) are slower in HFIP than in isopropanol. Surprisingly, the collective hydrogen-bond dynamics (probed by dielectric spectroscopy) in HFIP are significantly faster than in isopropanol.
The researchers conclude that in HFIP the hydrogen-bonded clusters are smaller and their collective rearrangements occur much faster than in isopropanol, whereas the individual hydrogen-bonds (the dynamics of which are reflected in individual molecular re-arrangements) are longer lived. These differences can be explained by the subtle balance between hydrogen-bond donor and acceptor strength: HFIP is a stronger hydrogen-bond donor, and a weaker hydrogen-bond acceptor, than isopropanol. This leads to smaller, but longer-lived hydrogen-bonded clusters in HFIP. The resulting larger number of free hydrogen-bond donor groups in HFIP, which can donate stronger hydrogen-bonds, can enhance reaction rates observed in HFIP.
The research was performed in a cooperation between the HIMS groups of Molecular Photonics (Sander Woutersen) and Synthetic Organic Chemistry (Tati Fernandez), the Polymer and Soft Matter Dynamics group at the Université libre de Bruxelles (Federico Caporaletti), and the Liquid Dynamics group at the Max Planck Institute for Polymer Research (Lucas Gunkel and Johannes Hunger).
Paper details
Federico Caporaletti, Lucas Gunkel, María Ángeles Fernández-Ibáñez, Johannes Hunger, Sander Woutersen: Fast Collective Hydrogen-Bond Dynamics in Hexafluoroisopropanol Related to its Chemical Activity. Angewandte Chemie International Edition, Accepted Article, e202416091.
DOI: 10.1002/anie.202416091