Repurposing inert amides for drug discovery
31 January 2025
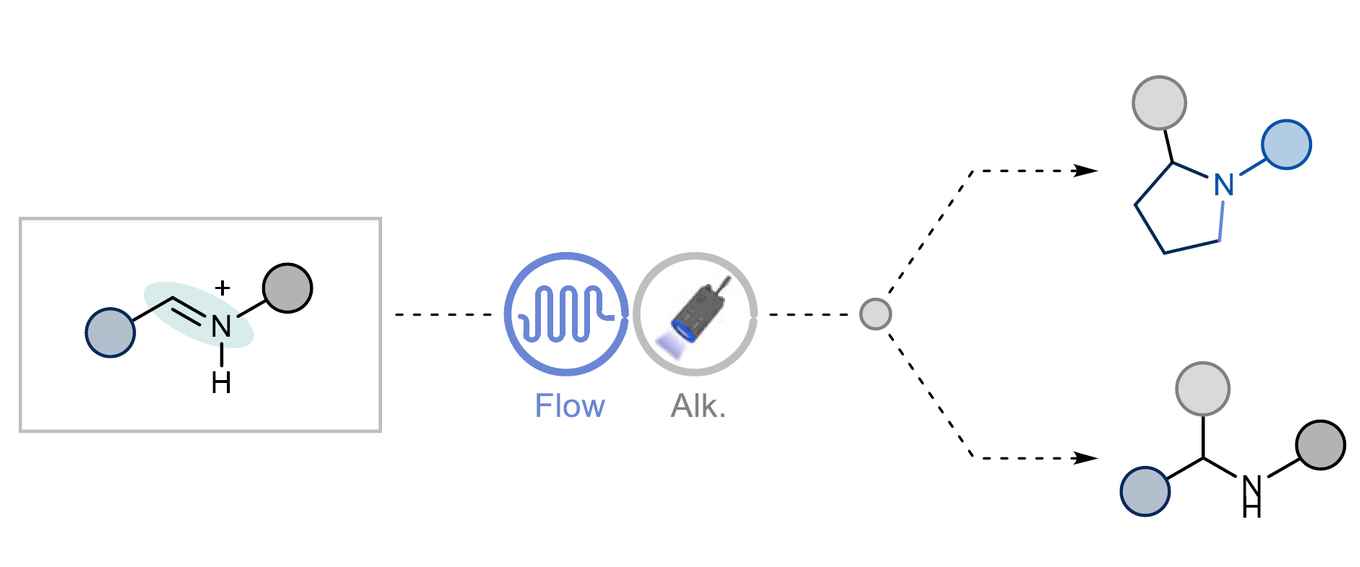
Mechanistic studies of the novel method confirmed the role of radical intermediates. The novel approach offers a broad functional group tolerance – compatible with diverse substrates – as well as scalability, which is enabled by process optimization and continuous-flow technology. The application in drug discovery particularly facilitates late-stage modifications of drug-like molecules.
Abstract, as published with the paper
Secondary amines are vital functional groups in pharmaceuticals, agrochemicals, and natural products, necessitating efficient synthetic methods. Traditional approaches, including N-monoalkylation and reductive amination, suffer from limitations such as poor chemoselectivity and complexity. Herein, we present a streamlined deoxygenative photochemical alkylation of secondary amides, enabling the efficient synthesis of α-branched secondary amines. Our method leverages triflic anhydride-mediated semi-reduction of amides to imines, followed by a photochemical radical alkylation step. This approach broadens the synthetic utility of amides, facilitating late-stage modifications of drug-like molecules and the synthesis of saturated N-substituted heterocycles. The pivotal role of flow technology in developing a scalable and robust process underscores the practicality of this method, significantly expanding the organic chemist’s toolbox for complex amine synthesis.
Paper details
Antonio Pulcinella, Stefano Bonciolini, Robin Stuhr, Damiano Diprima, Minh Thao Tran, Magnus Johansson, Axel Jacobi von Wangelin & Timothy Noël: Deoxygenative photochemical alkylation of secondary amides enables a streamlined synthesis of substituted amines. Nat Commun 16, 948 (2025). DOI: 10.1038/s41467-025-56234-w