Sustainable synthesis of formamides via a methylisocyanide intermediate
25 March 2025
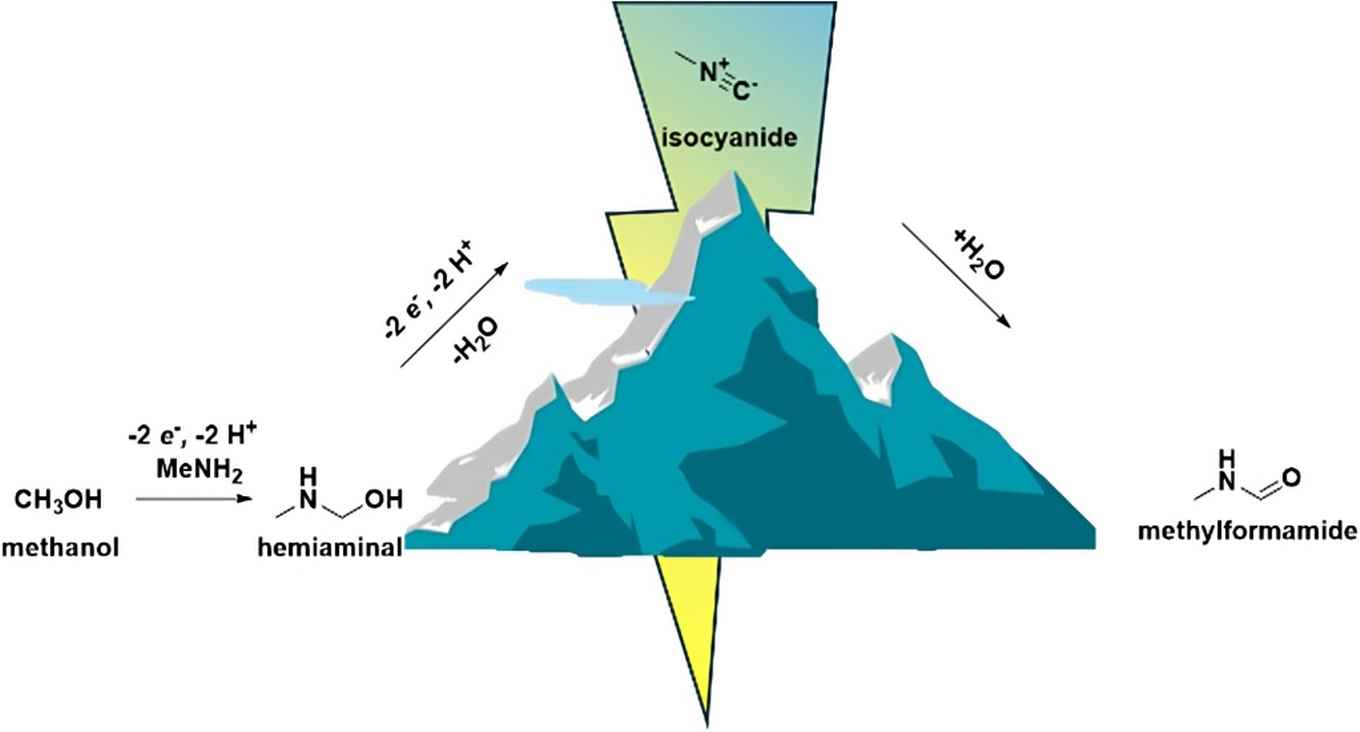
The discovery is rather unusual because isocyanides are not commonly observed in electrosynthetic processes. The researchers demonstrated the presence of methylisocyanide in a multicomponent reaction and confirmed its reactivity in situ.
In their JACS paper, they describe how the electrochemical formation of methylisocyanide and other reactive intermediates opens up new possibilities for safer and more controlled synthesis of a range of molecules.
Abstract, as published with the paper
Electrochemical methodologies offer a transformative approach to sustainable chemical synthesis by enabling precise, energy-efficient transformations. Here, we report the selective electrochemical N-formylation of methylamine using methanol as both reagent and solvent, facilitated by a simple glassy carbon electrode. Under optimized conditions, we achieve a faradaic efficiency (FE) of 34% for methylformamide synthesis in a neutral NaClO4 electrolyte. Mechanistic insights from in situ Fourier-transform infrared spectroscopy (FTIR) and complementary synthetic experiments reveal two distinct reaction pathways: the direct oxidation of a hemiaminal intermediate and a novel route involving the formation of methylisocyanide, which subsequently hydrates to yield methylformamide. The presence of methylisocyanide was confirmed through mass spectrometry analysis following a successful Ugi multicomponent reaction, demonstrating the ability to safely utilize reactive intermediates within an electrochemical framework. This work underscores the potential of electrosynthesis to unlock metal-free, sustainable pathways to produce value-added nitrogen-containing compounds, paving the way for greener approaches in chemical manufacturing and catalysis.
Paper details
Pim J. L. Broersen, Lars Wielhouwer, Gadi Rothenberg, Amanda C. Garcia: Electrochemical N-Formylation of Amines: Mechanistic Insights and Sustainable Synthesis of Formamides via a Methylisocyanide Intermediate. J. Am. Chem. Soc., published 12 March 2025. DOI: 10.1021/jacs.4c16725