Prof. Wybren Jan Buma receives HFML-FELIX funding
Characterising short-lived intermediates and mechanisms of catalytic conversions
25 November 2025
The funding, which enables Buma to appoint a PhD candidate, follows from the first call of the new Incentive Fund with which HFML-FELIX aims to strengthen its Research and Engineering Program. This features a large national partnership consisting of Radboud University (which hosts HFML-FELIX) and seven other academic partners, including the University of Amsterdam.
The proposed research aims to obtain key insights into catalytic reactive intermediates. Their structure and behaviour hold the key to understanding the mechanism(s) by which catalysts perform their function. This is crucial to their rational design and optimisation, and reaching the full potential of catalytic conversions. It will drive novel chemistry that can address many challenges of modern society in areas such as energy, health and sustainability.
Zooming in on neutral catalysts
By their very nature, since they are very short-lived and highly reactive, catalytic reactive intermediates are difficult to get a grasp on. While many studies have been performed on ionic catalytic species, studies on neutral catalysts are all the more difficult.
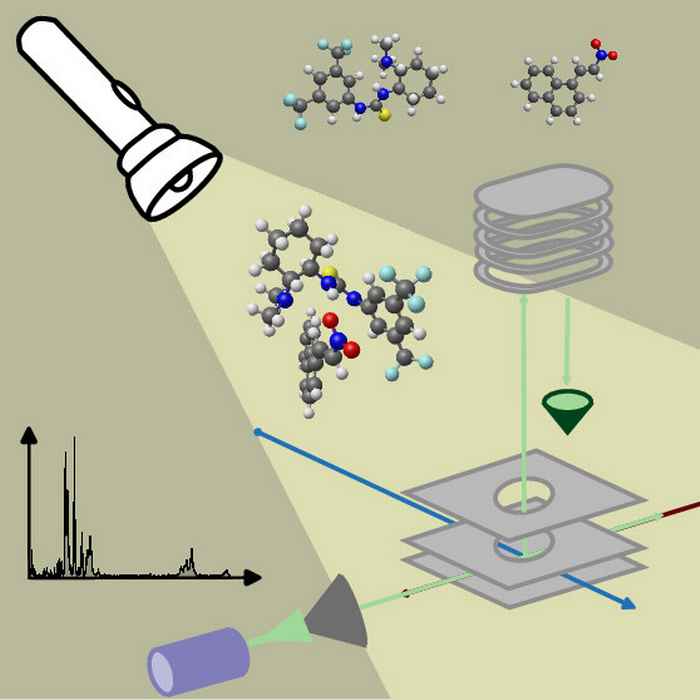
Here, Buma and co-workers have recently reported major breakthroughs using the advanced infrared spectroscopy facilities at HFML-FELIX. The research now funded by the Incentive Fund will build on this, investigating key catalytic cycles involving organo-, metal-organo-, and photocatalysts. Adding to the experimental results, detailed quantum chemical calculations will help elucidate the precise characteristics of the catalysts.
The research features collaboration of Buma with Dr Piero Ferrari at HFML-FELIX, as well as Dr Daria Galimberti at Radboud University’s Institute for Molecules and Materials and Prof. Lucas Visscher at Vrije Universiteit Amsterdam (Department of Chemistry and Pharmaceutical Sciences).