Safe and scalable synthesis of fluorinated amine compounds for medicinal chemistry
10 November 2025
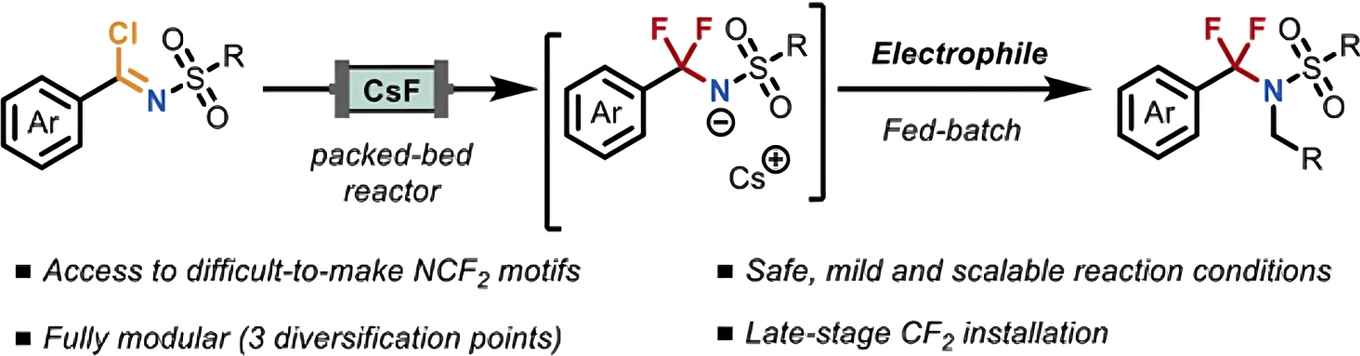
At the heart of the strategy is the on-demand generation of NCF2R anions using a packed-bed microreactor containing CsF. It features mild conditions, avoids hazardous fluorinating reagents and minimizes fluorinated waste. Leveraging three points of diversification, it allows efficient access to a broad range of NCF2R scaffolds. The method is compatible with complex drug-like scaffolds, making it a powerful tool for medicinal and fluorine chemistry.
Abstract, as published with the paper
The α,α-difluoromethylene amine (NCF2R) motif represents a useful functionality in medicinal chemistry, yet practical and modular methods to access this class of compounds are lacking. Here, we report a safe and scalable flow-based strategy for the on-demand generation of NCF2R anions using a packed-bed microreactor containing caesium fluoride. This protocol enables the late-stage installation of the CF2 group under mild conditions, avoiding the use of hazardous fluorinating agents and minimizing fluorinated waste. This fully modular strategy features three points of diversification (carboxylic acid, sulfonamide, and electrophile), allowing efficient access to a broad range of α,α-difluoromethylene amines. The method tolerates a variety of functional groups, supports late-stage functionalization of pharmaceutically relevant scaffolds, and is compatible with downstream cross-coupling reactions, demonstrating the robustness of the reaction protocol. This work provides a versatile platform for the streamlined incorporation of NCF2 motifs, expanding the range of synthetic strategies available in medicinal and fluorine chemistry.
Paper details
Nagornîi, D., Ronco, P., Anwar, K., Kaplaneris, N., Douglas, J. J., & Noël, T. Flow-Enabled, Modular Access to α,α-Difluoromethylene Amines. Angewandte Chemie International Edition, e17282. DOI: 10.1002/anie.202517282
The paper is included in the ‘hot topic’ collection of Angewandte Chemie papers in the field of Flow Chemistry