Light-driven decarboxylative trifluoroethylation of carboxylic acids
10 February 2026
The method was developed at the Flow Chemistry group led by Prof. Timothy Noël in collaboration with scientists of the specialty chemicals company Enamine. The reaction mechanism was elucidated in close collaboration with Prof. Bas de Bruin of the Homogeneous, Supramolecular and Bio-inspired Catalysis research group.
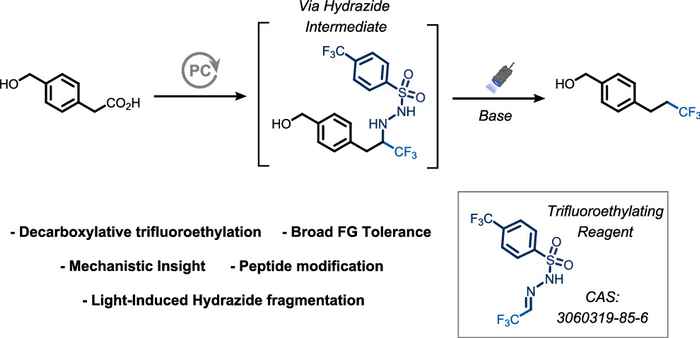
The selective incorporation of fluorine atoms or fluorinated groups is a key strategy for tuning lipophilicity, metabolic stability, membrane permeability, and binding affinity in modern medicinally relevant compounds and agrochemicals. The method presented in the paper adds to the toolbox of the synthetic chemist for introducing such groups. Conceptually, it can be viewed as a C₁-homologative trifluoromethylation, effectively expanding the scope for introducing a trifluoromethyl group and creating fluorinated building blocks that are often difficult or expensive to access. The reagent involved is already commercially available from Enamine.
Beyond scope and practicality, the work also led to an interesting mechanistic insight: the use of light instead of heat fundamentally changes the behaviour of the key hydrazide intermediate. In effect, photochemical conditions selectively favour the desired radical pathway.
Abstract, as published with the paper
The incorporation of fluorinated alkyl groups is a powerful strategy to fine-tune the physicochemical and biological properties of organic molecules. In particular, the trifluoroethyl (−CH2CF3) substituent offers a valuable C1-homologated analogue of trifluoromethylated motifs, yet methods for its direct introduction at sp3-hybridized carbon centers remain scarce.
Here, we report a general and practical approach for the decarboxylative trifluoroethylation of aliphatic carboxylic acids under near visible-light irradiation. The transformation proceeds via photoinduced generation of a carbon-centered radical that adds to a bench-stable sulfonyl hydrazone reagent derived from trifluoroacetaldehyde, followed by light-driven fragmentation to furnish the desired trifluoroethylated products. The reaction operates under mild conditions, exhibits broad substrate scope, including primary, secondary, and tertiary acids, and tolerates diverse functional groups.
Conceptually, the process can be viewed as a C1-homologative trifluoromethylation, offering a distinct retrosynthetic disconnection for the synthesis of trifluoroethyl-containing building blocks. Mechanistic studies combining experimental and computational analysis provide insight into the fragmentation behavior of the key alkylated sulfonyl hydrazide intermediate.
Paper details
Federico Belnome, Antonio Pulcinella, Stefano Bonciolini, Mattia Lepori, Oleksandr P. Datsenko, Zhen He, Matteo Gasparetto, Pavel K. Mykhailiuk, Bas de Bruin, and Timothy Noël: A C1-Homologative Trifluoromethylation: Light-Driven Decarboxylative Trifluoroethylation of Carboxylic Acids. J. Am. Chem. Soc. article ASAP, published 6 February, 2026. DOI: 10.1021/jacs.5c21423