Amsterdam aquatolide synthesis enables Science publication on NMR
Aquatolide produced in the Hiemstra lab serves as testing compound for new robust NMR analysis
11 April 2017
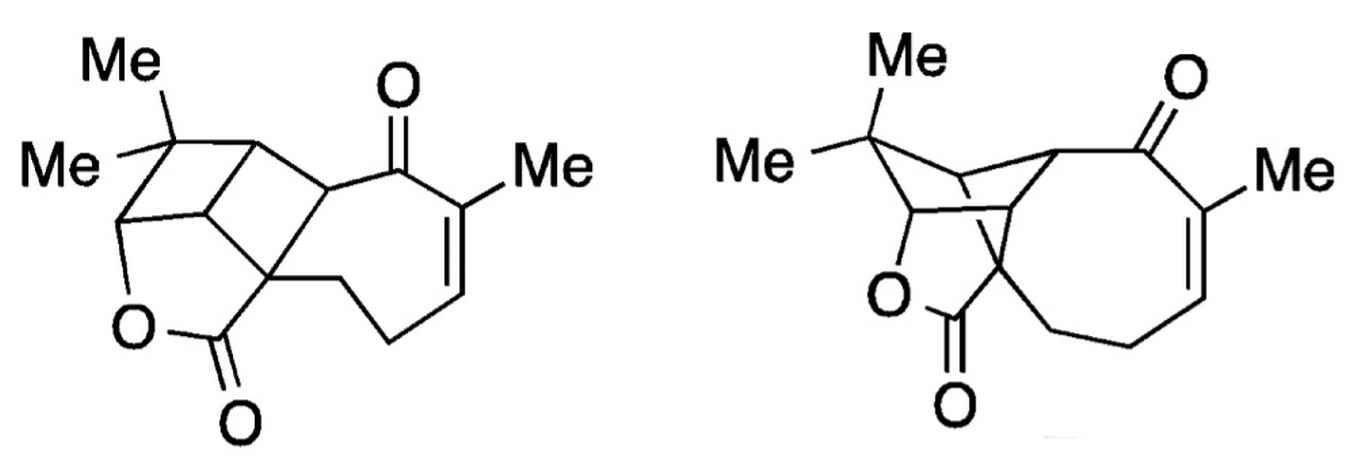
Establishing the molecular structure of complex chemical compounds by means of nuclear magnetic resonance (NMR) spectroscopy can be quite troublesome. The interpretation of the spectroscopic data often involves educated guesswork, which can from time to time result in an incorrect structure determination.
Aquatolide provides an interesting example here. In 1989 the structure of this natural compound, found in the Asteriscus aquaticus plant, was established by NMR analysis, although erroneously. It took researchers more than 20 years to discover this mistake and in 2012 a new research team reported the correct aquatolide structure.

New NMR method
In a recent Science publication researchers of the Structure Elucidation Group at the Merck company together with scientists of Harvard Medical School and the University of Connecticut present a new, robust method for analysis of NMR data. To demonstrate the power of their method they use the aquatolide case and show that their approach (using so-called anisotropic NMR parameters) underpins the 2012 determination of the compound's structure.
This crucial part of their NMR analysis could not have been performed without the chemical curiosity of professor Henk Hiemstra of the University of Amsterdam's Van 't Hoff Institute for Molecular Sciences.
Synthesis of 16 steps

It was in Hiemstra's lab that the synthesis of aquatolide was developed yielding the high quality samples for the recent NMR analysis. When the 2012 structure of the compound was published, Hiemstra noticed the similarity to solanoeclepin A, a hatching agent of potato cyst nematodes. Since the Hiemstra lab had been working on a method for synthesis of solanoeclepin A, he set out to use the 'synthetic toolbox' of his lab for the synthesis of aquatolide.
Thanks to the perseverance of MSc student Jordy Saya and lab technician Roel Klein Nijenhuis the team achieved a 16 steps synthesis, reported in Organic Letters in 2015. It attracted immediate attention from NMR researchers requesting samples, which Hiemstra was happy to provide.
Although he did not actively participate in the research currently published in Science, the NMR researchers valued the contribution of his lab of such importance that Hiemstra was listed as co-author. "It's my first Science publication, and in all probability also my last", says a proud and happy Hiemstra, who will retire early next year.
Publications:
Current publication in Science:
Yizhou Liu, Josep Saurí, Emily Mevers, Mark W. Peczuh, Henk Hiemstra, Jon Clardy, Gary E. Martin, R. Thomas Williamson: Unequivocal determination of complex molecular structures using anisotropic NMR measurements. Science Vol. 356, Issue 6333, eaam5349. DOI: 10.1126/science.aam5349
Aquatolide synthesis in Organic Letters:
Jordy M. Saya, Klaas Vos, Roel A. Kleinnijenhuis, Jan H. van Maarseveen, Steen Ingemann, and Henk Hiemstra: Total synthesis of Aquatolide. Org. Lett., 2015, 17 (15), pp 3892–3894 DOI: 10.1021/acs.orglett.5b01888