Promiscuous enzyme enables efficient and benign synthesis of nitriles
First report of direct enzymatic transformation of alcohol to nitriles
28 September 2018
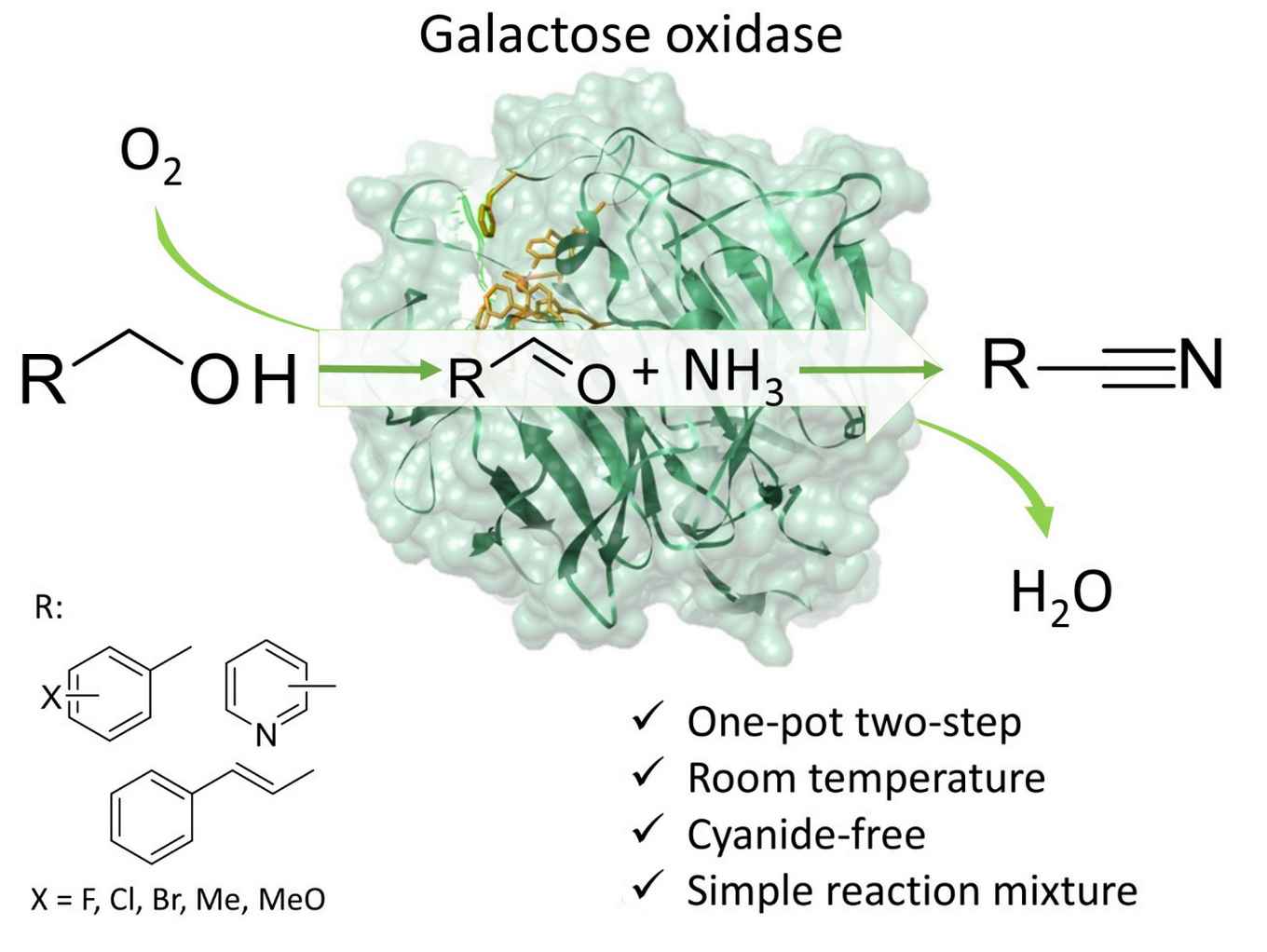
Researchers Jan Vilím, Tanja Knaus and group leader Francesco Mutti of the HIMS Biocatalysis research group foresee potential application of their discovery in for instance the synthesis of cinnamonitrile, an important synthetic fragrance, and of cyano-pyridines that are – among others – used as precursor in the synthesis of vitamin B3. Moreover, the industrial manufacturing of pharmaceuticals can benefit from the new enzymatic method because it can efficiently convert benzylic alcohols into benzonitrile moieties that constitute the active core of a significant number of pharmaceuticals. The new biocatalytic method enables a more efficient synthesis as it requires only molecular oxygen and ammonia, and avoids the use of toxic cyanide.

A serendipitous discovery
The Angewandte Chemie paper by the HIMS-Biocat researchers is the first report of a direct enzymatic transformation of alcohol to nitriles. The promiscuous formation of nitriles catalysed by purified galactose oxidase enzyme was discovered by chance, during the study of the enzymatic oxidation of benzyl alcohol to benzaldehyde using plain air as oxidant. When the researchers performed this oxidation in an ammonium formate buffer, a tiny amount of benzonitrile could be detected.This immediately sparked their interest. They set out to optimize the benzonitrile production and achieved satisfying yields under mild conditions (pH 9, 30 °C, air at atmospheric pressure) albeit with rather moderate turnover numbers. However, switching from purified enzymes to cell free extracts from Escherichia coli bacteria, they were able to achieve a turnover number (TON) up to ca. 3300, which is a value already suitable for large scale application.
Industrial potential
The researchers subsequently tested the promiscuous enzymatic conversion of alcohols into nitriles with a variety of substrates. It was established that three main families of alcohols can be converted into nitriles using galactose oxidase. Adding to this, the researchers expect that other enzymes comparable to galactose oxidase might show similar catalytic activity but on different type of alcohols. They therefore see great potential for their biocatalytic conversion as an alternative for current industrial nitrile synthesis methods.The advantage of the use of enzymes lies in the absence of toxic compounds (notably cyanide) and the use of an aqueous environment. Furthermore, the conversion proceeds under atmospheric pressures and requires less energy consumption (for heat and pressure) in comparison to chemical methods. Adding to this, no chemical method for the conversion of alcohols into nitriles has a comparable atom efficiency, which means that the biocatalytic method generates less waste. Increasing the conversion above 99% and maximizing chemoselectivity would lead to a reaction mixture of pure nitrile and eliminate the need for further purification steps, which has been already achieved with selected substrates.

Optimization and expansion
Because of the importance of the discovery and the quality of the research, the Angewandte Chemie editors consider the paper 'highly important', which places it among the top 10% publications of the renowned journal. The HIMS-Biocat researchers are now investigating two research sub-lines to further develop their discovery. On one hand, they are screening for nitrile formation with other naturally occurring copper-dependent alcohol oxidases that are already known to convert other substrates in the natural reaction (the oxidation of alcohols to aldehydes). Protein engineering of galactose oxidase and other copper-dependent oxidases will also help to expand the substrate scope of the reaction.
On the other hand, they are working on further optimization of the nitrile forming reaction with the already discovered galactose oxidase in order to achieve higher turnovers. Their approach is chemical stabilization of the biocatalysts as well as recycling of the biocatalyst in subsequent batch reactions or even its application in flow reactors. This research programme has the potential to lead to a wide exploitation of copper-dependent oxidases for the sustainable manufacturing of nitriles in the fine chemical industry.
Publication details
Jan Vilím, Tanja Knaus, Francesco Mutti: Catalytic Promiscuity of Galactose Oxidase: A Mild Synthesis of Nitriles from Alcohols, Air and Ammonia. Angewante Chemie International Edition. DOI: 10.1002/anie.201809411