Chris Slootweg in Nature Chemistry: ‘Green Chemistry is an outdated concept’
UvA researcher introduces twelve principles of future Circular Chemistry
21 February 2019
Slootweg is dissatisfied with the Green Chemistry concept that currently dictates sustainability thinking in the chemical industry. Presented exactly 20 years ago by two chemists from Yale and MIT, the twelve principles have become the proverbial 'Ten Commandments' of responsible chemistry. ‘However’, Slootweg argues, ‘Green Chemistry represents 'old thinking' since it optimises a linear production chain whereas future sustainable processes should be circular.'
According to Slootweg, the concept of Circular Chemistry expands the scope of sustainability to the entire life cycle of chemical products. ‘We have to replace today's linear, take-make-dispose approach with circular chemical processes and work towards a closed-loop, waste-free chemical industry.’
Repurposing indefinitely

By adhering to the twelve principles of Circular Chemistry now presented in Nature Chemistry by Slootweg and former chemistry Master's students Tom Keijer and Vincent Bakker, the industry will optimise resource efficiency across chemical value chains. ‘The concept of a circular economy is aimed at extending rather than wasting the inherent value of materials embodied in a product’, says Slootweg. ‘Chemistry is crucial for achieving this. By making chemical processes truly circular, products can – ideally – be repurposed almost indefinitely, using energy as only input.’
Through these twelve principles, says Slootweg, Circular Chemistry offers a new paradigm. Life cycle thinking and circularity will reinvent chemistry and provide the basic principles for developing novel chemical products and processes that use waste as a resource. ‘This will contribute to realising the circular economy and secure our sustainable future by addressing the United Nations’ Sustainable Development Goals.’
Publication details
Tom Keijer, Vincent Bakker, and J. Chris Slootweg: Circular Chemistry to enable a Circular Economy. Nature Chemistry, Volume 11, March 2019., 190-195. DOI: 10.1038/s41557-019-0226-9
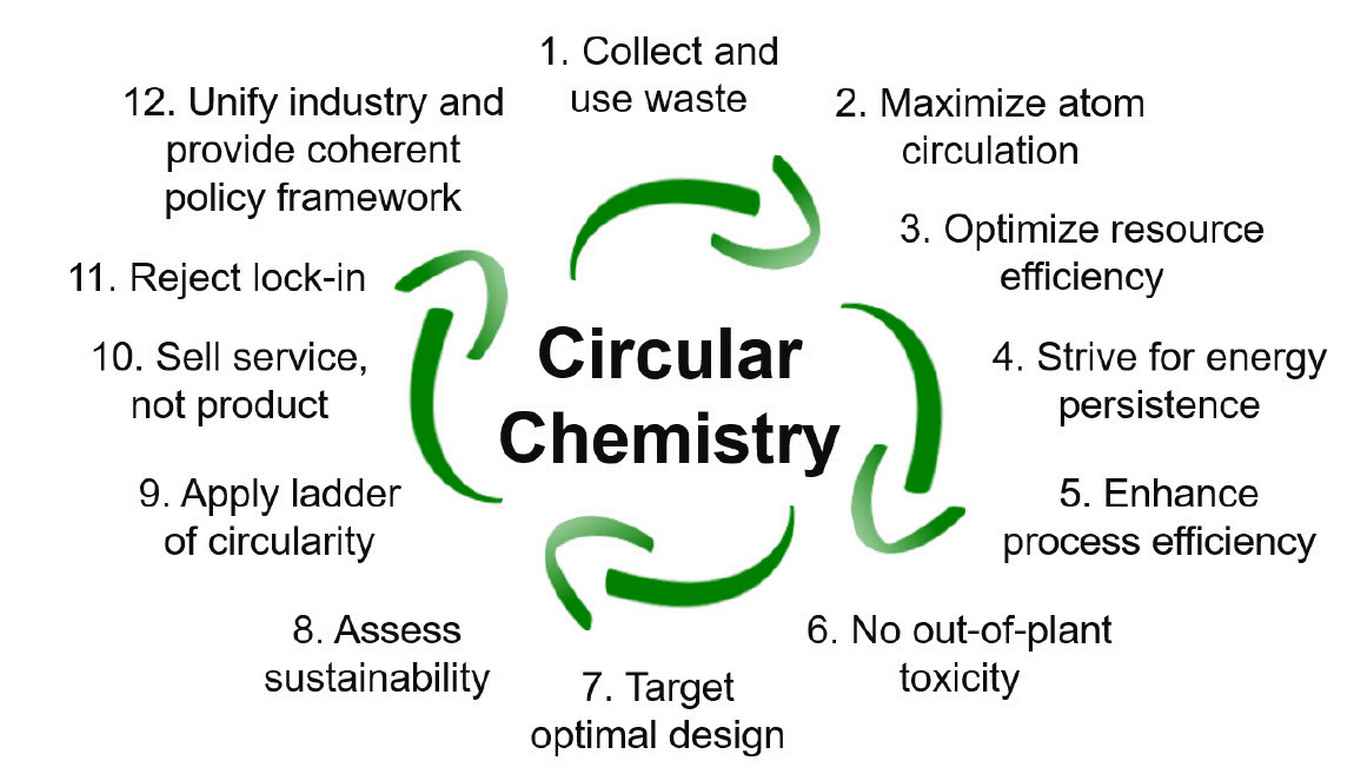
The twelve principels of circular chemistry
1. Collect and use waste
Regarding waste as a resource is a prerequisite for circularity. Redirecting waste streams and their use as chemical feedstock should become ubiquitous for the synthesis of marketable products, to achieve complete recirculation of molecules and materials.
2. Maximize atom circulation
Circular processes should aim at maximizing the utility of all atoms in existing molecules. This requires optimal product design which allows for efficient separation and purification, and enhances reuse and recycling opportunities in an environmentally benign way.
3. Optimize resource efficiency
Currently renewable resources offer the chemical industry an opportunity to diversify its raw materials base, but often bio-based materials are created in a linear production process without sustainable end-of-life options. In Circular Chemistry, materials are continuously cycled back through the value chain for reuse, optimizing resource efficiency and preserving finite feedstocks.

4. Strive for energy persistence
For repurposing waste material as a feedstock, it is important to realize recirculation of molecules and materials of the highest bond energy, thereby retaining as much of their renewable energy input as possible to maximize energy efficiency. By viewing the energy stored in materials as long-term investment, Circular Chemistry realizes conservation of energy and reduces additional energy inputs.
5. Enhance process efficiency
Innovations are required that promote both the recycling (separation) of materials during the chemical process (in-process) and the repurposing of used products (post-process), using chemical or biochemical methods that reduce primary feedstock use and optimize resource yields.
6. No-out-of-plant toxicity
Circular Chemistry strives to reduce the harmful impact of chemicals. The use of substances of concern may be unavoidable on-site but they should not be released to the environment. This requires the continued conglomeration of industrial sites and enhanced cooperation between companies.
7. Target optimal design
Product design should target the most favourable end-of-life, avoiding persistence in the environment and breakdown into harmful products. As an example, although biodegradable materials are often seen as sustainable, it is often overlooked that after disposal partly degraded polymers can cause more harm to the environment than the plastic bottle itself. It is therefore preferable to collect any waste stream and convert it in dedicated plants to value-added materials.
8. Assess sustainability
Environmental assessments, typified by the life cycle assessment (LCA), should become prevalent to identify inefficiencies in current chemical processes and evaluate the sustainability performance throughout the entire life cycle of a chemical product.
9. Apply the ladder of circularity
The ladder of circularity (reject, reduce, reuse, redistribute, repair, refurbish, repurpose, remanufacture, recycle, recover, return) provides a means to assess the end-of-life options for a product. Circular Chemistry aims for the highest possible forms of recycling, accounting for separation, purification and degradation to sustain chemicals and processes.
10. Sell service, not product
A service-based chemical industry is vital to Circular Chemistry, as companies have the assets and the know-how to retrieve and repurpose chemical products and are equipped to target the management of molecule-circulating loops. This will lead to the more efficient use of chemicals, and to improved health and safety, environmental, and economic benefits.
11. Reject lock-in
Circular Chemistry innovations need to promote transitions and overcome lock-ins to realize market opportunities for long-term sustainability ambitions. Business and regulatory environment need to be flexible and allow the implementation of innovations.
12. Unify industry and provide coherent policy framework
The industry and policy should be unified to facilitate the adoption, development and implementation of Circular Chemistry. A supportive policy framework is required to ensure that the value chain is balanced over the whole cycle of materials circulation.