Versatile and fast all-visible-light molecular switch achieves breakthrough in molecular photocontrol
UvA research provides first confirmation
3 June 2019
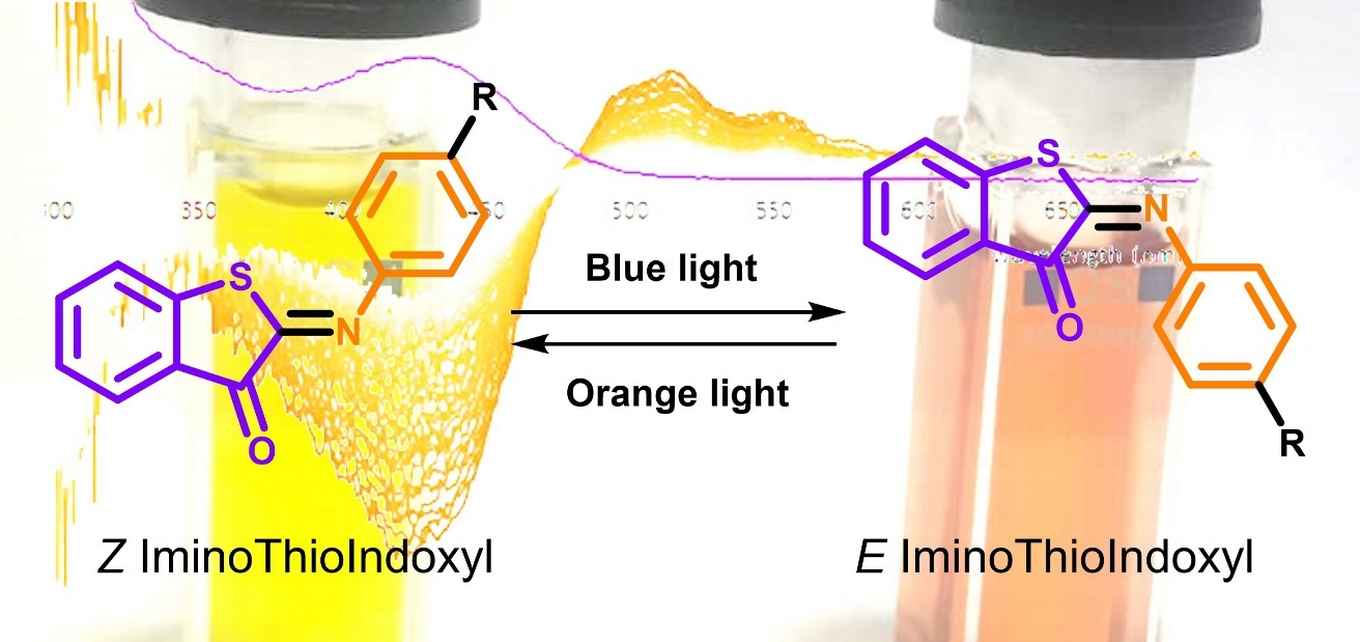
In our macroscopic world, we are used to being able to switch a device on or off. Such control is also one of the ‘holy grails’ at the molecular scale. Light-activated molecular photoswitches are in this respect of particular interest. They allow, for example, for a non-invasive and localized means to activate a drug precisely where and when it is needed. However, existing switches are far from ideal as they require harmful ultraviolet light for their operation rather than harmless visible light. This is a show-stopper from a medical settings point of view. Furthermore, they cannot exclusively be switched from one state to the other, and normally do not function under physiological conditions of the human body.
HIMS research confirms successful 'molecular crossbreeding'
Currently, many molecular switches make use of thioindigo and azobenzene chemical motifs, albeit that they suffer from the drawbacks mentioned above. Looking for improvements, Dr Wiktor Szymanski of the University Medical Center Groningen (UMCG) realized that a fusion of these two motifs should also be able to function as a photoswitch. He expected that similar to crossbreeding, the resulting iminothioindoxyl (ITI) molecule would very likely have improved properties compared to its 'parents'. The initial results were, however, quite disappointing. ‘We didn’t see any change in the absorption spectrum when we irradiated it', says Mark Hoorens, the UMCG PhD student who synthesized the new compound and tried to switch it. 'Nothing seemed to happen. We lost interest and went on with other research’.
Things changed for the better when the Groningen researchers discussed their results with Michiel Hilbers and Wybren Jan Buma of the HIMS Molecular Photonics group, at the 2017 International Symposium on Photopharmacology in Groningen. It seemed worthwhile to repeat the iminothioindoxyl irradiation experiments using the HIMS facilities, which have a better time resolution.

Indeed, this produced surprising results. Hoorens and Hilbers at first did not believe their eyes. ‘We saw a completely separated absorption band appear 100 nm to the red of the steady-state absorption band of ITI, with a lifetime of about 10 to 20 milliseconds', says Hilbers. 'At first we even suspected that we were looking at a contamination in the sample’.
Nothing could be further from the truth. The Amsterdam experiments had confirmed the success of Szymanski's concept of molecular crossbreeding. One of the 'parents' of ITI absorbs in the UV region and has band separation, while the other absorbs in the visible light region but lacks a good band separation. The new switch has the best of both worlds, resulting in properties that have never before been observed in a photoswitch.
Further confirmations
Follow-up experiments confirmed that ITI is indeed the fully-visible-light switch the scientists were looking for. Experiments on a femto- and picosecond timescale performed in the laboratories of Dr Mariangela Di Donato at the European Laboratory of Non-Linear Spectroscopy allowed for further mechanistic studies. According to Di Donato: ‘From these studies it became clear that ITI switches on an ultrafast timescale of a few hundreds of femtoseconds. This is similar to how fast the visual pigment in our eyes is switched when light falls on it'.
The final confirmation was provided by quantum chemical calculations performed by Dr Adèle Laurent (University of Nantes) and dr. Miroslav Medved’ (Palacky University in Olomouc). These calculations predicted absorption maxima of the two photo-isomers that were very similar to those observed experimentally, but also a barrier for switching back to the original form that fitted excellently the observed lifetime. ‘In the first instance, we were quite puzzled by this gigantic 100 nm band separation’, say Laurent and Medved’, ‘but our calculations now provide a logical explanation for this. What is even better, is that they allow us to predict how ITI can be modified to meet specific requirements of its users’.
ITI: an incredibly versatile switch
Mark Hoorens has by now synthesized several varieties that have been further characterized in Amsterdam, Florence, Nantes and Olomouc. From these studies, it has become clear that ITI is an incredibly versatile switch that can be operated under a wide variety of experimental conditions including, importantly, biological ones, and with properties that are relatively easy to tune.
Reference
Mark W.H. Hoorens, Miroslav Medved', Adèle D. Laurent, Mariangela Di Donato, Samuele Fanetti, Laura Slappendel, Michiel Hilbers, Ben L. Feringa, Wybren Jan Buma & Wiktor Szymanski: Iminothioindoxyl as a molecular photoswitch with 100 nm band separation in the visible range Nature Communications 10, Article number: 2390 (2019); DOI 10.1038/s41467-019-10251-8