New simple device greatly improves analysis of reaction kinetics
"It's a little like having the world's first telescope or microscope"
4 November 2019
Measuring the kinetics of chemical reactions is common in many laboratories. Knowing the kinetics gives insight into systems as they move towards equilibrium. It's essential for testing hypotheses, for understanding reaction mechanisms, and for designing and optimizing chemical processes. But the task itself is often mundane, time-consuming, and labour-intensive. This is especially true when determining Arrhenius relations, which require multiple sets of measurements.
Now, reporting in Angewandte Chemie, PhD student Thierry Slot, working on the TOP-PUNT project Catalysis in Confined Spaces, presents a solution. He has built a simple device, based on bubble counting, to replace the century-old experimental set-up of the upside-down burette. Together with supervisors Dr Raveendran Shiju and Prof. Gadi Rothenberg, the prototype was developed into a bona fide piece of equipment, which is now used routinely in the lab.
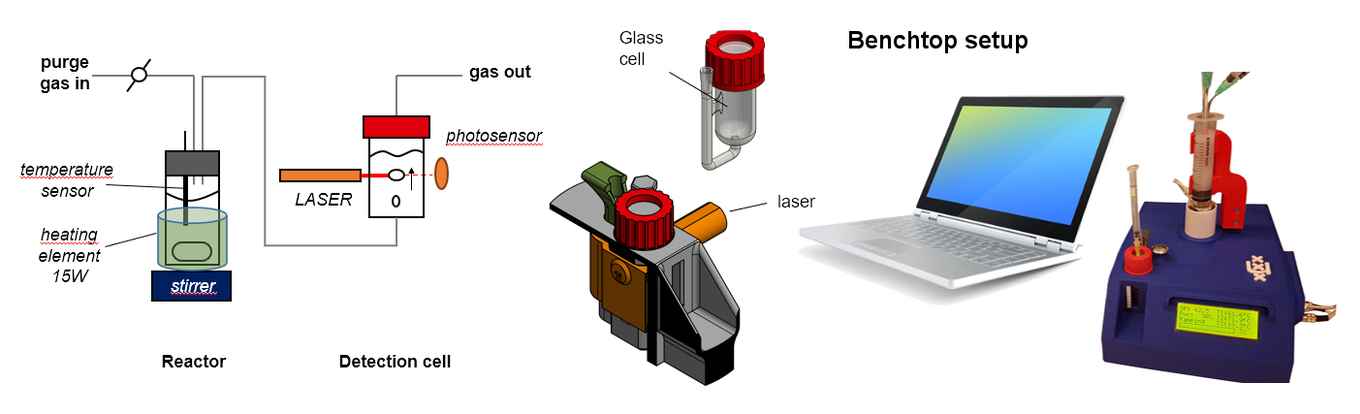
Slot built a first working prototype of the device out of frustration, to save time and hassle: “The upside-down burette is used to quantify the formation of gaseous products by measuring their volume. However, identifying small differences in reactivity requires the determination of rather small differences in volume, which always gave me problems. So I came up with the idea of a bubble counter that measures the development and passage of gas bubbles rather than the displacement of water in the upside-down burette. I built the first working prototype over a weekend, using some old parts scavenged from a gas chromatograph, a heating element and the laser from my cat’s toy."
Benchtop device
Rothenberg and Shiju immediately recognized the potential of the new method, and the group developed the bubble counter into a benchtop device enabling a sensitive and reliable quantification method.
Using a laser beam, the device detects the bubbles that arise as a result of the delicate balance between pressure and surface tension in the detection liquid. The unique feature of the device is its accurate timing of the gas bubbles, enabling a precise calculation of the volume of each bubble. This yields detailed and reproducible kinetic profiles with thousands of measurements in a single experiment. The device also includes a digital temperature controller, enabling the analysis of thermodynamic parameters and the precise calculation of Arrhenius relations.
Rothenberg sees additional value in this device for mechanistic studies: "The ability to measure very large numbers of data points is a game-changer because it can give insight to mechanistic features that were previously undetectable. It's a little like having the world's first telescope or microscope, a piece of equipment that enables you to see things beyond the state-of-the-art".
Open-access knowledge sharing
The researchers considered patenting and commercialising the device, but decided instead, after consulting with UvA's technology transfer office, to make it publicly available and open-access. Rothenberg, who has already founded three spin-off companies, is in favour of knowledge valorisation, but also weighs carefully the pros and cons in each case: "Many labs could benefit from this kind of device, but this doesn't immediately justify investing time, money and effort in commercialisation. Filing patents is simple. Getting value from patents is much harder. In this case, we chose to share the knowledge (including all of the files needed for 3D printing and manufacturing) so people can build such devices in their own labs".
Meanwhile, Thierry Slot has built two more devices for specific applications, which researchers in the group are already using. Several other labs are also looking on with interest.
Publication details
T.K. Slot, N.R. Shiju and G. Rothenberg: A simple and efficient device and method for measuring the kinetics of gas-producing reactions. Angew. Chem. Int. Ed., 2019, EarlyView. DOI: 10.1002/anie.20191100