Loading-dependent water effects on the structural properties of water stable MOFs
2 December 2019
Metal-organic frameworks (MOFs) are a class of crystalline, nanoporous materials formed by the assembly of inorganic nodes and multitopic organic linkers. Their exceptionally high porosity and tuneable structural properties make them a promising platform for technologies ranging from gas separation and storage to catalysis, chemical sensing and drug delivery. Understanding of the chemical stability and guest-host interactions of MOFs under practical operating conditions is essential to the design and development of these materials.
In the current edition of the journal Nature Chemistry researchers from the Georgia Institute of Technology (Atlanta, USA) and the Van 't Hoff Institute for Molecular Sciences (HIMS) at the University of Amsterdam (The Netherlands) contribute to this understanding with a joint study of the effects of water loading on the structural properties of a water-stable MOF. The research entailed a series of in situ synchrotron X-ray diffraction experiments and infrared spectroscopy performed in the group of Prof. Krista Walton at Georgia Tech, and molecular simulation analysis by PhD student Jurn Heinen and Dr David Dubbeldam from the HIMS computational chemistry group. In Nature Chemistry, the researchers show that although the MOF under study does not show any large-scale structural transitions upon interactions with water, it does have a dynamic and reversible structural response to water guest molecules.
Dynamic structural change
The MOF under study was DMOF-TM, a functionalized zinc metal-organic framework that is an isostructural variant of the prototypical DMOF-1 framework that displays low-pressure CO2 affinity and high water stability. These characteristics are due to the presence of methyl groups on the terephthalate ligand that do not prevent water from entering the pore space but nonetheless play a critical role in improving the material’s moisture stability. Understanding the exact origin of this increased stability has important broader implications for MOF crystal engineering strategies using intelligent linker design to improve chemical stability and functional properties.
In Nature Chemistry, the researchers present their findings on how the DMOF-TM structure adapts to the exposure to water. They observe changes in the unit cell parameters, microstrain, vibrational spectra, and atomic structure. Importantly, these changes manifest themselves at low water loading and continue to change in response to this loading. Combining these observations with molecular modelling, the researchers propose dynamic structural changes of the MOF structure in response to H2O guests molecules as an explanation for the water stability of DMOF-TM. They present a scenario (shown in the figure below) where bond rearrangement occurs in the presence of water but does not result in irreversible structural changes or degradation.
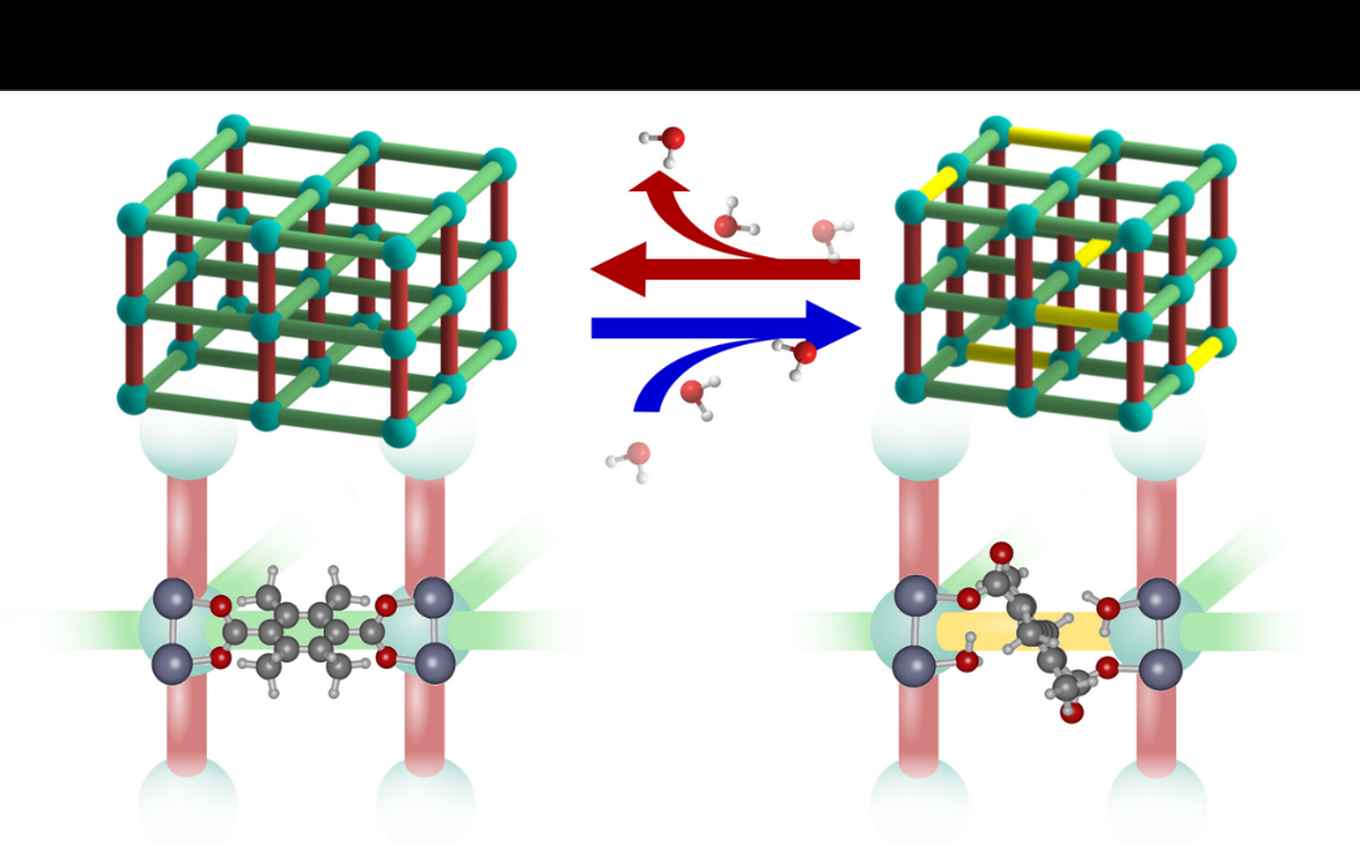
The study thus highlights the fact that even for MOFs that are highly stable in humid environments a static representation of the framework structure may not be sufficient to understand the observed stability. It illustrates the importance and complexity of guest-host interactions with respect to the stability of MOFs and underpins the importance of a detailed study of MOF structures even in the absence of large-scale structural transitions.
Publication details
Nicholas C. Burtch, Ian M. Walton, Julian T. Hungerford, Cody R. Morelock, Yang Jiao, Jurn Heinen, Yu-Sheng Chen, Andrey A. Yakovenko, Wenqian Xu, David Dubbeldam, Krista S. Walton: In-situ visualization of loading-dependent water effects in a stable metal–organic framework. Nature Chemistry, DOI: 10.1038/s41557-019-0374-y
Dr Nicholas C. Burtch: https://orcid.org/0000-0003-0052-7307
Dr Jurn Heinen: https://www.uva.nl/en/profile/h/e/j.heinen/j.heinen.html
Dr David Dubbeldam: https://www.uva.nl/profiel/d/u/d.dubbeldam/d.dubbeldam.html
Prof. Krista Walton: https://walton.chbe.gatech.edu