Synthesis of chiral amines in neat organic solvents using immobilised enzymes in a continuous flow reactor
19 March 2020

Chiral amines are important molecules in chemistry, notably for the synthesis of active pharmaceutical ingredients (APIs). In industry, they are typically produced in batch processes that involve the use of specialised catalysts. However, such processes are not always very effective and often result in unwanted by-products. As an alternative, the field of biocatalysis explores the catalytic use of natural or nature-derived (i.e., engineered) enzymes that offer more atom-efficient and selective synthesis, thereby enhancing product yield and reducing waste production.
Inactivity in neat organic solvents
An important drawback of most enzymes, however, is the relatively poor applicability or even inactivity in neat organic solvents. These solvents can be preferred over water in many chemical syntheses as they improve the dissolution of hydrophobic starting materials, thus enhancing conversion rates. While some enzyme families—particularly hydrolases—have been investigated and adapted for their use in non-aqueous environments, the so-called aminotransferase enzymes that are very relevant for the synthesis of α-chiral amines could thus far only be used in aqueous environments. They have only sporadically been used in non-aqueous media albeit with lower productivity and efficiency.
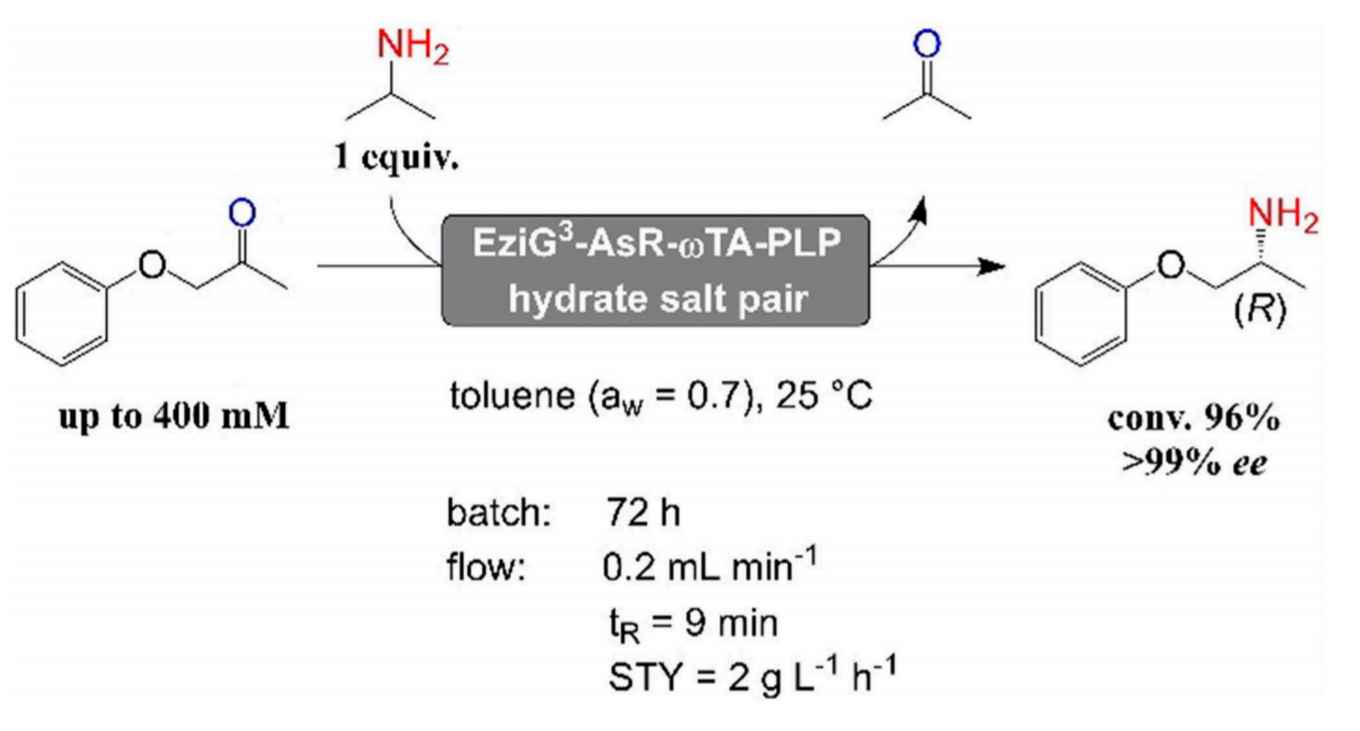
The research on ω-aminotransferases of the HIMS Biocatalysis group led by Dr Francesco Mutti now changes that. PhD student Wesley Böhmer, who graduated late 2019, developed a method for the efficient biocatalytic synthesis of α-chiral amines using immobilised ω-aminotransferases in neat organic solvents. In a paper designated as Very Important Publication and accompanied by a front cover graphic in the recent edition of Advanced Synthesis & Catalysis, Böhmer and Mutti, together with industrial collaborators from EnginZyme AB, explain the method and report its potential for industrial application in a continuous flow reactor.
Salt hydrates to control water activity
The method features aminotransferase enzymes immobilised on porous glass beads, resulting in enhanced enzyme stability and a large surface area per unit of volume. In previous research, the HIMS researchers already reported very high conversion rates using this immobilised enzyme system in an aqueous environment, in particular regarding the kinetic resolution of racemic α-chiral amines using pyruvate as ketone acceptor. These results were published a little over a year ago.
Now, they have been able to adapt the immobilised enzyme system for the synthesis of α-chiral amines from a ketone and an amine donor in neat organic solvents; this work was carried out in collaboration with researchers at EnginZyme AB in Sweden that engineered the carrier materials for enzyme immobilisation. Since dynamic and catalytic properties of enzymes in non-aqueous media are dependent on the presence of a limited but critical amount of water, the Amsterdam group applied salt hydrates during both the biocatalyst immobilisation step and the progress of the reaction. The reaction system was investigated in terms of carrier material, organic solvents and reaction temperature. Optimal conditions were found with more hydrophobic carrier materials and toluene as reaction solvent. Under batch conditions, a chemical turnover (TTN) above 13000 was obtained over four subsequent reaction cycles, which is already a remarkable value for the challenging asymmetric reductive amination of ketones.
Perspective on industrial application
The applicability of the immobilised biocatalyst in neat organic solvents was further demonstrated in a continuous flow packed-bed reactor. This showed excellent performance without observable loss of enzymatic catalytic activity over several days of operation. In general, ca. 70% conversion was obtained in 72 hours using a 1.82 mL flow reactor. The conversion rate rose above 90% when the reaction was run up to 120 hours. According to the researchers, these results pave the way for scale-up towards a demonstration reactor under industrially relevant conditions. Although this would require adjustments and improvements, the research now published in Advanced Synthesis & Catalysis represents an important first step towards this goal.
Publication details
Wesley Böhmer, Alexey Volkov, Karim Engelmark Cassimjee, and Francesco G. Mutti: Continuous Flow Bioamination of Ketones in Organic Solvents at Controlled Water Activity using Immobili ω-Transaminases. Advanced Synthesis & Catalysis DOI: 10.1002/adsc.201901274
Link
Website HIMS Biocatalysis research group.