Controlling the rate of heterogeneous electron transfer across the rim of nanospheres
Paper in the Journal of the American Chemical Society
20 April 2020
Abstract
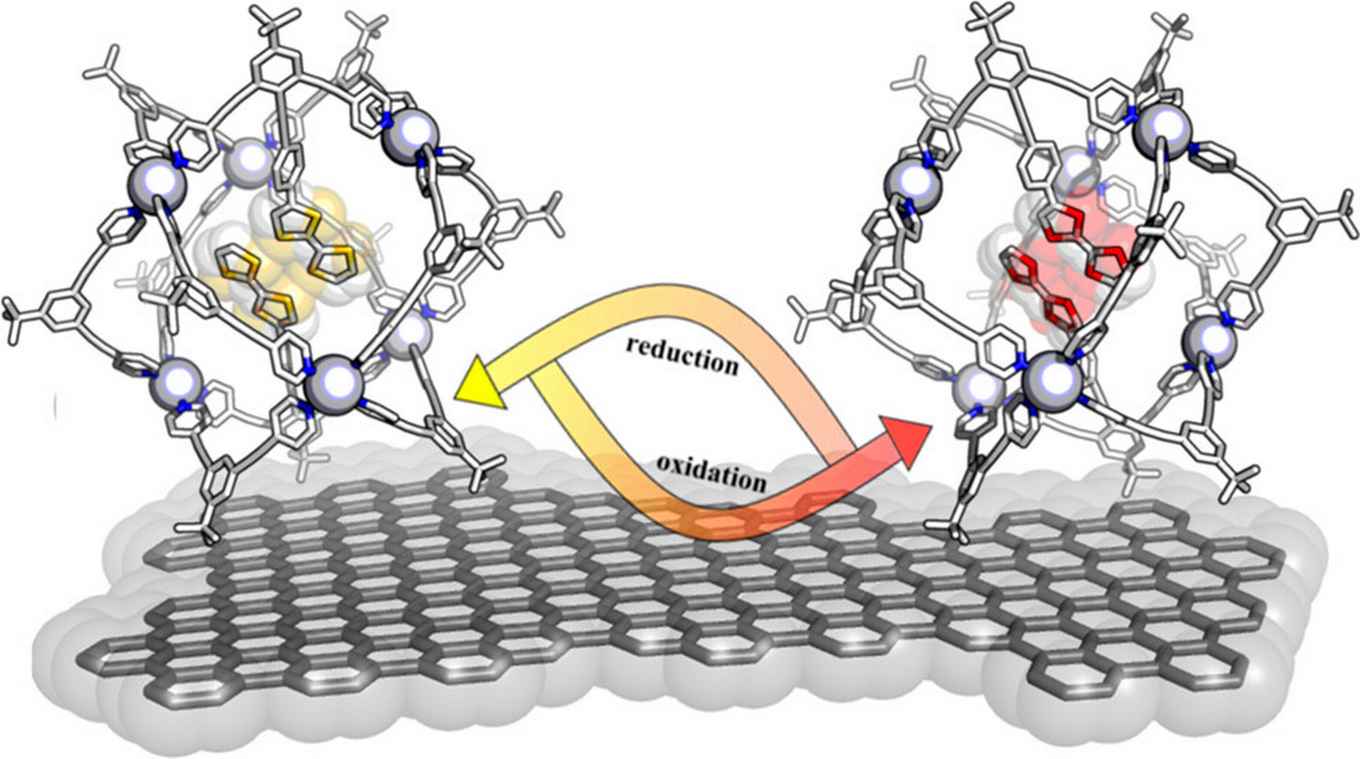
Catalysis in confined spaces, such as those provided by supramolecular cages, is quickly gaining momentum. It allows for second coordination sphere strategies to control the selectivity and activity of transition metal catalysts, beyond the classical methods like fine-tuning the steric and electronic properties of the coordinating ligands. Only a few electrocatalytic reactions within cages have been reported, and there is no information regarding the electron transfer kinetics and thermodynamics of redox-active species encapsulated into supramolecular assemblies. This contribution revolves around the preparation of M6L12 and larger M12L24 (M = Pd or Pt) nanospheres functionalized with different numbers of redox-active probes encapsulated within their cavity, either in a covalent fashion via different types of linkers (flexible, rigid and conjugated or rigid and nonconjugated) or by supramolecular hydrogen bonding interactions. The redox probes can be addressed by electrochemical electron transfer across the rim of nanospheres, and the thermodynamics and kinetics of this process are described. Our study identifies that the linker type and the number of redox probes within the cage are useful handles to fine-tune the electron transfer rates, paving the way for the encapsulation of electroactive catalysts and electrocatalytic applications of such supramolecular assemblies.
Paper
Riccardo Zaffaroni, Eduard O. Bobylev, Raoul Plessius, Jarl Ivar van der Vlugt, and Joost N. H. Reek: How to Control the Rate of Heterogeneous Electron Transfer across the Rim of M6L12 and M12L24 Nanospheres J. Am. Chem. Soc. 2020, 142, 19, 8837–8847 DOI: 10.1021/jacs.0c01869